Abstract
Gene flow can provide cohesion between conspecific populations. In order to obtain an indirect measure of gene flow between coral reef species in the eastern tropical Pacific (ETP) and between these populations and those of the rest of the Pacific we compiled available data from sequences of DNA and microsatellites for corals, gastropods, echinoderms and fishes, and calculated FST statistics. The ETP consists of a narrow strip of continental shelf along the coast of the Americas and a deeper water gap between the coast and the outer eastern Pacific Islands; a large expanse of deep ocean separates the ETP and the closest islands in the central Pacific. We have, therefore, compared populations in four major directions: (1) between the eastern and the central Pacific, (2) between the coast and the outer islands, (3) among the outer islands, and (4) along the coast and nearshore islands. The available data are biased in favor of showing high levels of gene flow because they contain an excess of transpacific species, which are a minority among ETP biota. Despite this bias, shallow water populations of the ETP are isolated from the rest of the world’s oceans. Occasional breaching of the expanse of water between the ETP and the Central Pacific by some species is also possible. Gene flow between the outer eastern Pacific islands and the mainland coast is variable, depending on the species examined. Gene flow among populations at the outer eastern Pacific islands is high except for those at Easter Island (Rapa Nui), in which all but one sampled species show large and significant values of FST in comparisons with populations from all other islands. Gene flow rates among populations along the ETP coast are high. There is no evident genetic break resulting from the Central American Gap (southern Mexico to the Gulf of Fonseca, Honduras) in any of the sampled species. A trend of isolation by distance along the coast is evident in corals and fishes.
Similar content being viewed by others
Keywords
16.1 Introduction
Gene flow is the exchange of genes between conspecific populations. It is an important biological process in every species because gene transfer provides cohesion between populations, preventing their independent evolution. The movement of genes over generations assures that new advantageous mutations that arise in one location can eventually spread throughout the species’ range. When gene flow becomes restricted populations become “genetically structured”, diverging from each other and (if the restrictions become severe and last a long time) possibly turning into separate species. The sharing of genes between populations can also dilute adaptation to local environmental conditions, thus averaging the response to natural selection over the connected populations. It may thus limit the geographic extent over which a species can spread, as peripheral populations are prevented from adapting to conditions at the edge of a species range and then proceed to colonize new, previously unsuitable, areas (Haldane 1956; Case and Taper 2000; Sexton et al. 2009; Dawson et al. 2010).
Genetic exchange is effected by the dispersal of propagules from one population to another, but, as propagules are difficult to follow, most estimates of gene flow do not involve direct observations of such transfers; they rely instead on assessing divergence between populations. This is particularly true for marine organisms in which planktonic larvae maintain genetic connections over very long distances and are virtually impossible to track in the water column. Species with this kind of larvae are expected to show high rates of genetic exchange between populations and thus high genetic homogeneity, although this expectation is not always met. Several studies have indicated that in a number of marine species self-recruitment to the natal population is high, and the dispersal potential expected of their larvae fails to be realized (Jones et al. 1999; Swearer et al. 1999, 2002; Hellberg et al. 2002; Taylor and Hellberg 2003). Such studies have led to doubts that the dogma of near-panmixia in marine organisms is ever realized (Hellberg 2009). Even when local recruitment does occur, however (as it undoubtedly does in most populations), and even if the fraction of the larvae that recruit successfully at a distant locality is small, it may be sufficient to homogenize genetic constitution of populations over large distances.
The position of coral reef organisms of the eastern tropical Pacific (ETP) in the spectrum between panmixia and high genetic structure is the subject of this chapter. We begin by considering geographical and oceanographic features that would be expected to affect rates of contemporary and historical gene flow, then summarize existing data from the literature about genetic connectivity. Not all of the data were gathered to assess population structure, and the organisms for which genetic data exist are far from uniformly spread over taxonomic groups. Nevertheless, a compilation of such data can begin to address the question of whether general patterns exist. In “coral reef organisms” we include all species that are represented by populations resident in coral reefs, even if they are not exclusively found in this particular habitat. As most of these species (even corals) are capable of living outside coral reefs (Robertson 1998; Guzmán et al. 2004), this means that we have attempted to include studies on all organisms that live on hard substrata in the photic zone, except for those limited to the upper intertidal shore line.
16.2 Oceanographic Features of the Tropical Eastern Pacific Relevant to Gene Flow
The ETP (Fig. 16.1) consists of a narrow strip of continental shelf along the coast of the American continent with some islands close to the shore, a deeper water gap between the coast and the outer eastern Pacific Islands (Easter Island, the Galápagos Archipelago, Malpelo Island, Isla del Coco, Clipperton Atoll and the Revillagigedo Archipelago). A large expanse of deep ocean separates the ETP and the closest islands in the central Pacific (the Line Islands, the Marquesas, and Hawaii). Thus, shallow water populations in the ETP can exchange genes in four major directions: (1) between the eastern and the central Pacific, (2) between the mainland coast and the outer islands, (3) among the outer islands, and (4) along the coast and nearshore islands. Potential barriers to gene flow in each direction consist of habitat unsuitable for the establishment of adult populations and of currents that channel the movement of larvae along a particular vector, or that alter environmental conditions as to exceed the tolerances of propagules in a particular area (see Chaps. 3 and 4, Fiedler and Lavín, and Wang et al., respectively). Expanses of deep water that are difficult to cross in a single larval life can be expected to cause the most marked restrictions to the exchange of genes. If impassable, these barriers can prevent the spread of species, thus establishing different biogeographic provinces as determined by patterns of species presence and absence.
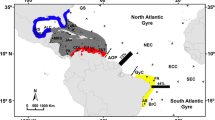
Ten year mean (1993–2003) ocean surface currents in the Central-East Pacific (a) and the Eastern Tropical Pacific (b). Given are surface current vectors with 1 degree (a) or 1/3 degree (b) resolution. Basemap generated with ETOPO 2 (USGS). CC = California Current, NEC = North Equatorial Current, NECC = North Equatorial Counter Current, SEC = South Equatorial Current, CRCC = Costa Rica Coastal Current
Physical barriers that impede gene exchange between populations in the ETP and those in the rest of the world define it as a separate oceanic region. Towards the east, an uninterrupted land bridge has existed for the last 2–3 million years (Coates and Obando 1996). [It has been claimed recently that a nearly complete barrier to water exchange with the Caribbean has existed since the Eocene or early Miocene (Montes et al. 2012) but this claim is incompatible with paleoceanographic (Keigwin 1982; Collins 1996; Haug and Tiedemann 1998; O’Dea et al. 2007), paleontological (Webb 1976; Coates and Obando 1996), and genetic (Lessios 2008) data.] Towards the west lies the widest marine biogeographical barrier on the planet, 4000–7000 km of deep water without any stepping stone habitats on which adults of shallow water marine species can exist (Ekman 1953; Briggs 1974). This ocean configuration, known as the “Eastern Pacific Barrier” (EPB) has been in place for most of the Cenozoic (Grigg and Hey 1992). It is so difficult to cross, that most shallow water benthic genera are represented by different species on its two sides. Had it not been for the small number of “transpacific species”, species that span the EPB (Emerson 1978, 1982; Vermeij 1978; Rosenblatt and Waples 1986; Lessios et al. 1996; Robertson et al. 2004; Lessios and Robertson 2006), it would not have figured in a discussion of conspecific gene flow. The barrier is traversed by the westerly North and South Equatorial Currents and by the easterly North Equatorial Counter Current. The speed of the latter increases greatly during El Niño events, reducing the transit time between the Line Islands and the eastern Pacific to periods that may not exceed the larval duration of a number of organisms (Richmond 1990; Glynn et al. 1996; Glynn and Ault 2000; Robertson et al. 2004). This acceleration of the North Equatorial Counter Current is generally proposed as the conveying mechanism for the dispersal of species from the species-rich central Pacific into the ETP (Dana 1975; Richmond 1990; Robertson et al. 2004) and may also be responsible for recurrent events of gene flow, at least in one direction.
Expanses of deep water narrower than the EPB are also of potential relevance to genetic divergence between conspecific populations of the outer islands of the eastern Pacific and the mainland. Easter Island (Rapa Nui), 4000 km from the coast of Peru, is the most remote of these islands. As its distance from Pitcairn is 1700 km, it can either be considered as the westernmost island of the eastern Pacific or the easternmost island of the central Pacific. Its marine fauna consists of a mixture of endemic, Indo-West-Pacific, transpacific, and cosmopolitan species, but it also contains several species characteristic of the eastern Pacific (Fell 1974; Rehder 1980; Randall 1998; Glynn et al. 2007). The latter may be the result of larval transport in currents that flow predominantly towards the west (Glynn et al. 2007). The next most remote island, Clipperton Atoll, lies inside the tropics, 1000 km from the coast of Mexico and 4000 km from the Marquesas Islands. It is intermittently bathed by either the easterly North Equatorial Counter Current or the westerly North Equatorial Current and may well represent a stepping stone between the central and eastern Pacific (Glynn et al. 1996; see Chap. 5, Glynn et al.). As at Easter Island, the marine fauna of Clipperton is a mixture of Indo-West and east Pacific species, and also endemics (Glynn et al. 1996; Lessios et al. 1996; Robertson and Allen 1996). The Galápagos Islands comprise the only oceanic Archipelago in the Equatorial eastern Pacific. San Cristóbal, the closest island to the mainland, lies 930 km west of the coast of Ecuador. The Galápagos Islands contain the best studied marine biota among those of the outer islands (e.g., Bowman 1966; Glynn and Wellington 1983; James 1991; Grove and Lavenberg 1997). The marine fauna contains numerous species that are also found on the west coast of America, several species endemic to the eastern Pacific outer islands or just to the Galápagos, and a few Indo-Pacific species (Briggs 1974). The Archipelago is influenced by the Peru Oceanic Current flowing towards the Galápagos from the mainland, the North Equatorial Counter Current bringing water from the central Pacific, the South Equatorial Current flowing in the opposite direction, plus a southerly flowing current during the dry season out of the Panama Bight (Ab bott 1966; Glynn and Ault 2000; Kessler 2006). Isla del Coco is situated 690 km northeast of the Galápagos and 500 km west of Costa Rica. Its marine fauna consists mostly of eastern Pacific species, but there is a small number of outer island endemics and of Indo-Pacific species (Hertlein 1963). The four Revillagigedo islands lie approximately 390 km southwest of the southern tip of Baja California. The shallow water marine species on these islands are mostly a subset of those found on the mainland, but some Indo-Pacific species are also present. The corals show affinities with those of Clipperton (Glynn et al. 1996; Glynn and Ault 2000; Ketchum and Bonilla 2001). The Revillagigedo Archipelago is influenced both by the northerly Costa Rica Current and by the southerly California Current (Glynn and Ault 2000; Kessler 2006; see Chap. 3, Fiedler and Lavín).
Marine populations of the coastal ETP are expected to show more genetic connectivity than those of island populations. This coast, however, is not entirely without potential barriers. Hard substrate bottoms are interrupted by river estuaries and mangrove areas, unsuitable as habitats for adults of many coral reef species. A 1000 km stretch from southern Mexico to the Gulf of Fonseca at the Honduras-El Salvador border (the Central American Gap) is devoid of coral reefs or rocky bottoms, except for a small Pocillopora reef in Los Cóbanos, El Salvador (Glynn and Ault 2000; Reyes-Bonilla and Barraza 2003; but see Chap. 5, Glynn et al.). Wind-driven upwelling in the Bay of Panama, the Gulf of Papagayo, and at Tehuantepec (Kessler 2006) may also exclude some species with narrow thermal tolerances, such as Acanthaster planci (Glynn 1974). Separate biogeographic provinces along the American coast recognized by some authors (Briggs 1974; Veron 1995; Hastings 2000; but see Robertson and Cramer 2009; Briggs and Bowen 2012) attest to the existance of barriers to dispersal that prevent a number of species from spreading along its entire length. Major currents may be of less importance to dispersal along the coast compared to tidal flux and eddies, likely to spread larvae over moderate distances. Nevertheless, larval connectivity might be influenced by the general circulation pattern from south to north between approximately 20oN and 20oS (Kessler 2006; Fiedler and Lavín (Chap. 3)). The northerly Peru Oceanic and Coastal currents reach almost to the Panama Bight; in this area a reversing gyre moves water towards the north or towards the south, depending on the season. From Costa Rica to Baja California, the current flow along the coast is northward, all the way to the entrance of the Sea of Cortez (Gulf of California). The cold California Current flows southward on the west side of Baja California and limits colonization of most tropical species, thus defining the northern termination of the ETP.
16.3 Data on Gene Flow Between Conspecific Populations in the Eastern Pacific
We have attempted to compile all the existing data published until 2013 that can be used to calculate rates of gene flow among ETP populations of coral reef organisms. Most of these data consist of mitochondrial DNA (mtDNA) sequences. The mtDNA of corals, however, evolves at slow rates and does not provide information useful in comparing conspecific populations (Shearer et al. 2002; Hellberg 2006). Population genetic research of corals has, therefore, had to rely on either sequences of ribosomal internal transcribed spacers (ITS) or microsatellites . We have not included data from isozyme studies because the original data are not readily available, and because many of the organisms for which isozyme data exist have been revisited by studies involving DNA, less subject to the problem of “hidden variation”. Most of the studies that generated the data were not designed to address the question of gene flow within the eastern Pacific; because of this, the sample sizes for each local population are often smaller than necessary for robust statistical analysis. Nevertheless, sequence data from only a few individuals may present a not too distorted view of gene flow (Pluzhnikov and Donnelly 1996). Some of the published studies have included summary statistics that could be used directly. When they did not, we calculated these statistics from raw data downloaded from GenBank. Some studies had to be omitted because authors have not included locality information with the accessioned sequences, making it impossible to use their data to calculate gene flow between local populations.
In order to quantify degree of divergence relevant to gene flow, we used FST statistics (Wright 1951). FST is the ratio of genetic variance between populations over the pooled variance within populations. There are two, qualitatively different, ways of calculating FST statistics. One, as originally conceived by Wright, is based on the frequency of alleles. The other is relevant only to DNA sequences, and is based both on the frequency of haplotypes and on the number of nucleotide differences between them (Hudson et al. 1992). This measure is sometimes referred to as ΦST. We have used frequency-based FST for microsatellite data and ΦST for sequence data. As most of the published studies have not applied corrections to either of these values, we avoided using them in what we calculated from raw data.
FST is far from a perfect measure of gene flow. Under an island model (in which migration and genetic drift are at equilibrium, and in which migration rates and effective population sizes are equal between all demes), FST can be used to estimate the number of propagules dispersed from one population to another per generation (Wright 1951). These conditions, however, are rarely met in any species (Whitlock and McCauley 1999), and much less so in marine populations, which are open to immigration of larvae with potentially different genetic constitutions in each generation (Johnson and Black 1984). An additional problem with FST, particularly for frequency-based data, is that high within-population heterozygosity can create the appearance of no differentiation between populations, even if they share no alleles (Hedrick 2005; Hellberg 2007, 2009). ΦST can assume negative values if within-population variation is higher than between-population variation. Such negative values are incompatible with the notion that they estimate gene flow. Finally FST statistics are incapable of distinguishing between recurrent gene flow at low levels among recently separated populations and higher levels of gene flow between anciently separated ones (Hey and Nielsen 2004; Marko and Hart 2012). Despite these problems, FST is a useful index of genetic differences between populations over and above local variation, differences that increase with genetic isolation, and, unlike more sophisticated statistics of gene flow, it can be applied to all genetic markers. The latter advantage is the main reason why we use it in this chapter. We have arranged the data in tables organized along four axes in which populations can be compared, i.e. between the central and the eastern Pacific, between the outer (oceanic) islands of the eastern Pacific and the mainland, among the outer islands, and among populations distributed along the coast. This, of course, does not imply that the directions in which genes have traveled over the generations are necessarily limited to these axes.
16.3.1 Gene Flow Between the Central and the Eastern Pacific
As few species are shared between the ETP and the central Pacific, the number of studies comparing conspecific populations in the two areas is necessarily limited. Under the island model of Wright (1951), values of FST ≥ 0.2 (if calculated from nuclear markers) or ≥0.33 (if calculated from mtDNA) , which is haploid and maternally inherited) correspond to an estimate of less than one propagule per generation. Accordingly, FST values that approach these thresholds are held to indicate genetic exchange that cannot overcome the diversifying effects of genetic drift. By this standard, many of the FST values in Table 16.1 represent a high degree of divergence, indicative of low rates of gene flow over the enormous distances between shallow water habitats in the two oceans. The patterns of genetic exchange, however, differ between species, as would be expected if the EPB is sporadically breached by larvae carried during periods of acceleration of the North Equatorial Counter Current.
Combosch et al. (2008) compared sequences of the internal transcribed spacer (ITS) from the main reef frame-builder of the eastern Pacific, Pocillopora damicornis, with sequences from Hawaii and from the western Pacific. Divergence between populations at Hawaii and at Panama was high (Table 16.1) and approximately equal to divergence between populations from the eastern and the western Pacific. Combosch et al. (2008) attributed part of this divergence to introgression of genes from P. eydouxi and P. elegans into the genome of ETP populations of P. damicornis, facilitated by a shift from a brooding reproductive mode in the central and western Pacific to free-spawning of gametes in the ETP (Glynn et al. 1991). This conclusion has been challenged by Pinzón and LaJeunesse (2011), who regard all three morphospecies as a single genetic entity, and consider evidence of apparent hybridization to be artifacts of cloning and sequencing of ITS, due to its representation by multiple copies in the genome (see below). Despite this disagreement, the FST value between populations of Pocillopora on the two sides of the EPB is not large enough to suggest that what is regarded as P. damicornis in Hawaii is a different species than the eastern Pacific form. The planulae of P. damicornis contain phototrophic Symbiodinium endosymbionts, which permit them to generate energy during long-distance dispersal (Baird et al. 2009). Baird et al. (2009) have suggested that species with autotrophic larvae were the only ones capable of recolonizing the eastern Pacific after the formation of the Isthmus of Panama, following the extinction of the earlier western American coral fauna. Presumably, such colonization might have occurred during El Niño events, though there may be constraints in the period of development of zooxanthellate planulae at high temperatures (Yakovleva et al. 2009), which may limit the teleplanic advantages conferred by larval autotrophy.
The study of Porites by Forsman et al. (2009) included samples of the massive coral Porites lobata from Tahiti and the ETP. As expected, little gene flow was evident in ITS between the central Pacific and Easter Island, one of the most isolated islands in the world (Table 16.1). Surprisingly, however, divergence between populations from Tahiti and the Galápagos was very low, although significant. The low FST value in this comparison may be due to non-equilibrium conditions arising from a recent influx of larvae. In a study of the same species by Baums et al. (2012), based on 12 microsatellites , samples from Moorea were highly divergent from samples from four localities in the Galápagos Archipelago (Table 16.1). The extensive sampling in this study permits firm conclusions regarding gene flow in this widespread, broadcast spawning coral with autotrophic larvae. Gene flow in P. lobata is high within the ETP and within the central Pacific but severely restricted across the EPB, despite occasional low FST values between some populations from the Galápagos and some of the south central Pacific islands. Principal components and Bayesian STRUCTURE (Pritchard et al. 2000) analysis revealed that colonies at Clipperton Atoll group genetically with those from the central, rather than the eastern Pacific (Baums et al. 2012). Populations at Tabuaeran (Fanning Atoll) and Kiritimati were genetically less differentiated than those of other central Pacific islands from a number of locations in the ETP (Table 16.1).
The only genetic comparisons of transpacific molluscs are for two species of Conus. Duda and Lessios (2009) found that populations of C. ebraeus at Hawaii were highly differentiated in Cytochrome Oxidase I (COI) from two populations in the ETP (Table 16.1). It appears that Clipperton is a stepping stone to the rest of the ETP, because the sample from this atoll consisted of mitochondrial haplotypes also found in either Panama or Hawaii, plus an additional type otherwise only encountered in Okinawa. These three haplotypes were distantly related, suggesting that they did not evolve in situ, and that the Clipperton population is thus the result of haphazard, infrequent influx of larvae. Analysis of Molecular Variance (AMOVA) found high (ΦST = 0.263) and significant differentiation across the EPB (Duda and Lessios 2009). In contrast to those of C. ebraeus, COI haplotypes of Conus chaldaeus from Hawaii and Clipperton were very similar (Table 16.1; Duda et al. 2012).
Data from the corallivorous sea star, Acanthaster planci, illustrate that different conclusions about gene flow are sometimes drawn when different genetic markers are used. ETP populations of Acanthaster were originally described as a separate species, A. ellisii, based on morphological differences (Madsen 1955). Sampled by isozymes, their genetic constitution appeared to be similar to those of the central Pacific, which led Nishida and Lucas (1988) to the conclusion that there was only one species, connected by high gene flow across the EPB. When sequences of the mitochondrial gene COI were subsequently obtained from the entire range of Acanthaster (Vogler et al. 2008), they showed that mtDNA of the ETP populations belonged to the same lineage as that of populations from the rest of the Pacific. FST values between eastern and central Pacific localities, however, are very large in seven out of eight comparisons (Table 16.1), suggesting that there is no gene flow across the EPB.
A number of tropicopolitan sea urchin genera show the deepest divergences between central-west and eastern Pacific extant species (Lessios et al. 1999, 2001; McCartney et al. 2000). There are, however, two exceptions. Echinothrix is a genus that, until one of its species, E. diadema, was observed at Isla del Coco in 1987, was unknown from the ETP (Lessios et al. 1996). Sequencing of Cytochrome B (CytB) found a higher amount of divergence between populations of E. diadema at Isla del Coco and at Clipperton, on the same side of the EPB, than between these populations and those at Kiritimati and at Hawaii (Table 16.1). Lack of divergence between ETP and central Pacific populations is more likely to be the result of recent introduction than of recurrent gene flow. Lessios et al. (1996, 1998) suggested that these populations may have become established during the 1982–1983 El Niño, which also introduced a number of central-west Pacific species of fishes into the ETP (Robertson et al. 2004). The second case of a transpacific echinoid is that of Tripneustes. Despite doubts by some taxonomic authorities, T. gratilla from the Indo-Pacific and T. depressus from the ETP were regarded as separate species. Lessios et al. (2003), however, found that these two putative species shared the same mtDNA clade and are, thus, in all probability conspecific. Sequences of bindin, a nuclear gene responsible for sperm-egg recognition, led to the same conclusion (Zigler and Lessios 2003). Gene flow across the EPB in Tripneustes was restricted, as evidenced by high pairwise FST values between five ETP and three central Pacific locations (Table 16.1).
Fishes are the group that contains the highest number of transpacific species (Robertson et al. 2004), and have thus provided the greatest opportunities for assessing gene flow across the EPB. Data from fishes illustrate great diversity of evolutionary histories and gene flow rates, diversity that is consistent with “sweepstakes dispersal” between the oceanic regions on either side of the barrier. An isozyme study by Rosenblatt and Waples (1986) indicated little divergence between ETP and Hawaiian conspecific populations of twelve species. More recently, Lessios and Robertson (2006) compared two mitochondrial genes, ATPase8 and ATPase6, in twenty species considered as transpacific on the basis of morphology. Two of these turned out to be anciently separated between oceanic regions, as indicated by reciprocally monophyletic mtDNA clades, and thus should probably be recognized as separate species. Among the other eighteen, FST between central and eastern Pacific populations ranged from negative values to +0.94. The highest values, indicative of a complete cessation of gene flow, were those between demes of the surgeon fish Acanthurus triostegus sandvichensis from Hawaii and Johnston Atoll, and of A. triostegus triostegus from the ETP (Table 16.1), but also between A. triostegus sandvichensis and A. triostegus marquesensis from the Line Islands and the Marquesas (Lessios and Robertson 2006). It would appear, therefore, that there is genetic isolation between the Hawaiian-Johnston subspecies from the other two subspecies. These comparisons illustrate that populations at Hawaii are often isolated not only from those in the ETP, but also from the rest of the Pacific, as Baums et al. (2012) have also found in Porites lobata. Thus, data in Table 16.1 in which the central Pacific is represented only by samples from Hawaii may not be indicative of isolation between oceanic regions.
Divergence between populations of Acanthurus triostegus from Kiritimati and the Marquesas and from the ETP was generally high, but at the same time suggestive of low levels of gene flow, also evident in the sharing of the most common haplotypes (Lessios and Robertson 2006). Divergence between ETP and central Pacific localities was occasionally high (but inconsistent between comparisons of different populations) in the glass eye, Heteropriacanthus cruentatus, in the goatfish Mulloidichthys vanicolensis and in the squirrelfish Myripristis bendti. Such inconsistencies may well be the result of small sample sizes, because sampling of M. bendti CytB of some of the same localities by Craig et al. (2007) did not always produce similar FST values. Other species, such as the surgeon fish Acanthurus nigricans, the parrotfish Calotomus carolinus, the surgeon fish Ctenochaetus marginatus, the butterflyfish Forcipiger flavissimus and the moorish idol Zanclus cornutus showed practically no divergence across the EPB (Table 16.1), indicating that there has been either recent or recurrent gene flow. In an analysis of molecular variation (AMOVA) only two of the 18 transpacific species were found to have significantly higher differentiation between oceanic regions than within regions. FST and AMOVA cannot distinguish between on-going gene flow and recent isolation. For this reason, Lessios and Robertson (2006) employed “IM”, an algorithm of Bayesian estimation based on coalescence (Hey and Nielsen 2004), to deduce time of initial separation and degree and direction of subsequent gene flow. Such analyses do not always produce reliable results when they involve a single locus, but they can provide an indication regarding these parameters. Estimated time of initial separation between central and eastern Pacific conspecific populations ranged from 30,000 to 1,000,000 years ago, times more recent than the 3 million year final closure of the Isthmus of Panama (Coates and Obando 1996). These relatively recent estimates indicate that a vicariance scenario, according to which transpacific species are relicts of circumglobal connections that were severed by the rise of the Isthmus (McCoy and Heck 1976; Heck and McCoy 1978), is unlikely. Direction of gene flow deduced by IM was not always from West to East, as might have been expected if the North Equatorial Counter Current were the only means of conveyance. In eight out of the eighteen cases, migration was actually estimated as being higher in the opposite direction. Reconstruction of ancestral genotypes and comparisons of relative genetic diversity suggested that in at least two cases the original range expansion was from the ETP into the central Pacific. Thus, directions of initial colonization and subsequent gene flow do not always coincide.
In conclusion, marine shallow water biota of the ETP is, indeed, isolated from the rest of the world’s oceans, but breaching of the EPB is also possible. Such transpacific migrations of larvae most likely are the result of haphazard combinations of factors favorable for migration, such as timing of spawning relative to current speed intensifications, availability of rafting materials, and good fortune in encountering suitable habitat at the end of the dispersal event.
16.3.2 Gene Flow Between the Outer Eastern Pacific Islands and the Mainland Coast
Compared to the distances between the ETP and the central Pacific, those between the American mainland coast and the outer oceanic islands of the ETP (Revillagigedo, Clipperton, Isla del Coco, the Galápagos and Easter Island) are shorter, but far from negligible. The available data on gene flow across this 520–4000 km oceanic divide (Table 16.2) are biased towards showing high rates of migration because, by and large, they come from transpacific species likely to possess traits conducive towards high rates of dispersal.
Data from corals found on at least one island and on the mainland come from studies on Pocillopora and Porites. In corals, morphological plasticity (Todd 2008) and gene exchange between distinguishable morphs (Willis et al. 2006) contribute to uncertainties as to which populations belong to the same species. This is particularly true for Pocillopora in the ETP. Pinzón and LaJeunesse (2011) sampled individuals of this genus that by morphological criteria belonged to P. damicornis, P. verrucosa, P. capitata, P. meandrina, and P. eydouxi. They determined sequences of the internal transcribed spacer 2 (ITS2) and of an unidentified open reading frame in mitochondrial DNA , and they also genotyped seven microsatellite loci. According to all of these markers, Pocillopora in the ETP belong to three distinct clades, independently of morphotype. Microsatellites were the only markers sampled in both the islands and the mainland in Pocillopora types 1 and 3. They indicate little differentiation between islands and mainland in Pocillopora type 1 (the larger values between Revillagigedo and the mainland are based on only two specimens), but substantial divergence between the Galápagos and Panama in Pocillopora type 3 (Table 16.2). Pocillopora type 2 is endemic to Clipperton. The population of Porites lobata at Clipperton sampled with microsatellites by Baums et al. (2012) was strongly differentiated from populations on the mainland (Table 16.2). Populations from Isla del Coco and the Galápagos maintain high rates of gene flow with populations at the mainland, except for the one at the Ecuadorian coast.
There are high rates of genetic exchange between conspecific populations of echinoderms from the islands and the mainland (Table 16.2). Acanthaster planci from the ETP, though genetically differentiated from the same species from the central Pacific, was represented by identical COI haplotypes at Isla del Coco and in the Gulf of Chiriquí, Panama. All three species of sea urchins for which data exist were genetically homogeneous between the islands and the coast with one exception. Paradoxically, this exception consists of the transpacific species Tripneustes gratilla/depressus, in which populations at Clipperton and Easter Island maintain very little gene flow with populations along western American shores. Other sea urchin species in which larvae from the islands have not established viable populations on the mainland are Echinothrix diadema, which has yet to be observed outside Clipperton and Isla del Coco (Lessios et al. 1996), and Eucidaris galapagensis at the islands, which is reciprocally monophyletic in COI with respect to the continental E. thouarsii (Lessios et al. 1999). It is doubtful that larvae of these species are incapable of crossing between the islands and the continental shore; a more plausible cause of this pattern is that they fail to become established due to ecological factors.
Practically all the existing data regarding fishes at the oceanic islands of the ETP come from transpacific species, and, as expected, indicate high genetic connectivity with coastal populations (Table 16.2). The grouper Epinephelus labriformis, though endemic to the ETP, fits the same pattern. The wrasse Stethojulis bandanensis, however, shows a high degree of genetic isolation, both at Clipperton and at Isla del Coco. The cosmopolitan puffer Diodon holocanthus is the only fish species sampled from both Easter Island and the mainland; like Tripneustes, it illustrates that populations at this remote locality are genetically isolated from populations in the rest of the ETP.
Examination of FST values from species that have been sampled at more than one island suggests that populations at Easter Island and Clipperton Atoll are less connected to populations along the mainland (Table 16.2). Ideally we would like to test statistically whether there is a general trend showing that populations at geographically more remote islands are also genetically more isolated from conspecific coastal populations. As there are few islands, this cannot be done through correlation of genetic and geographic distances; it can be addressed, instead, by comparison of intraspecific divergence between the residents of each island and those of a common locality on the mainland. The only such comparison that provides a sample size sufficient for statistical testing is between populations from Panama and Clipperton, on the one hand, and between Panama and Isla del Coco, on the other. We compared FST values of the same nine species sampled in all three localities paired by genetic marker. The results indicate that, as expected from relative geographic distances, genetic isolation of populations at Clipperton from those at Panama was significantly higher than isolation of the same nine species at Isla del Coco (Wilcoxon paired sample test, p < 0.05).
16.3.3 Gene Flow Between the Outer Eastern Pacific Islands
Although genes are not necessarily transferred directly between the outer islands (mainland populations may act as stepping stones) it is useful to ask how genetically different island populations are from each other. The general picture in comparisons between island populations of various species (Table 16.3) is almost identical to the one presented by comparisons between the islands and the mainland (Table 16.2). There is high genetic connectivity between conspecific residents of most islands, except for those at Easter Island, in which all but one species show large and significant values of FST in comparisons with populations from all other islands. Clipperton Atoll shows a mixed pattern. Populations of Porites lobata, Tripneustes gratilla, the blenny Ophioblennius steindachneri and the wrasse Stethojulis bandanensis are very different from populations from all other islands. Populations of all other species, however, show high rates of gene flow.
16.3.4 Gene Flow Along the Coast of the Eastern Pacific
We would expect to observe the highest rates of gene flow along the American shores of the ETP. This is generally the case. Pinzón and LaJeunesse (2011) found no significant restrictions of gene flow in microsatellites of Pocillopora “type 1” in populations spanning approximately 4200 km from the Sea of Cortez to Panama (Table 16.4). Combosch and Vollmer (2011), on the other hand, reported that their microsatellite data of Pocillopora damicornis show significant structure at a much smaller scale on the Panamanian coast. This structure is not evident in FST values, in which only two comparisons between populations are larger than 0.073 (Table 16.4). It is somewhat more evident in RST [an FST equivalent that takes the step-wise mutation pattern expected from microsatellites into account (Slatkin 1995)]. AMOVA analysis found significant, but small variation between individual populations and also between populations that were grouped in three areas along the Panamanian coast. High rates of gene flow were found between microsatellite frequencies of most coastal populations of Porites lobata by Baums et al. (2012). One exception was the population at Ecuador, which is different from all populations at Costa Rica, though not from the one at Panama (Table 16.4).
In four species of sea urchins, genetic connectivity in mtDNA along the coast is generally high from Mexico to Panama (Table 16.4). As in Porites, the population of the echinoid Arbacia stellata in the southernmost periphery of the species is highly differentiated from those in the species’ center of distribution.
Among the fishes, those with transpacific ranges, Diodon holocanthus and Scarus rubroviolaceus, show no genetic structure (Table 16.4). This is hardly surprising in the case of the latter, because both sampling localities of this species are situated close to each other in the Bay of Panama, but in the case of Diodon they lie 4000 km apart on either side of the Central American Gap. Epinephelus labriformis also shows high genetic connectivity over long spans of the coast from Baja California to Panama. The case of the three species of the grunt Anisotremus studied by Bernardi et al. (2008) is somewhat surprising. Anisotremus interruptus and Anisotremus taeniatus, species usually found only in the proximity of hard bottoms, show no significant structure in either mitochondrial CytB or the nuclear S7 region over 4000 km of coastline. Anisotremus dovii, on the other hand, even though it prefers sandy and muddy bottoms (and should thus be more continuously distributed along the coast) appears to experience marked restrictions in gene flow between Mexico and Panama, at least in CytB. Only one out of the total twelve CytB sequences sampled in this species is shared between the two locations, which accounts for the high and significant FST value.
In contrast to the pattern of high gene flow along most of the tropical west coast of America, genetic connectivity within the Sea of Cortez can be quite low in a number of species. Gene flow in the ovoviviparous sea horse Hippocampus ingens was high among all populations sampled from Mexico to Peru by Saarman et al. (2010) except for one; the mitochondrial control region of the population from Guaymas in the Sea of Cortez was highly differentiated from that of every other population (Table 16.4). Sequences of the mitochondrial control region of the scarletfin blenny Coralliozetus micropes were reciprocally monophyletic between upper and lower Gulf regions (Riginos 2005), a level of divergence that is reflected in the FST values in Table 16.4. This is also the case in the Cortez triplefin Axoclinus nigricaudus (Table 16.4) and in the Gulf of California endemic sand bass Paralabrax maculatofasciatus (Stepien et al. 2001; Riginos 2005), but not in the redside blenny Malacoctenus hubbsi (Table 16.4). These genetic breaks were attributed by Riginos (2005) to a hypothetical Pleistocene deep water break of the Baja California Peninsula that bisected habitats of hard bottom fishes, as it did of terrestrial mammals and reptiles. IM analyses yielding similar times of divergence between upper and lower Gulf populations of five fish species supported the hypothesis that a historical barrier was responsible for present-day isolation patterns, but environmental differences might also be responsible (Riginos 2005).
Along a linear coast, such as that of the ETP, one would expect a pattern of isolation by distance (Wright 1943), as one population acts as a stepping stone for the dispersal of genes towards others down the line. To determine whether this was the case, we analyzed the data of all species in Table 16.4 for which more than three localities were sampled. We calculated correlations between FST values and geographical distance along the coast, using Mantel (1967) tests to estimate probabilities of the correlation coefficient. For this analysis, negative values of FST were replaced by zero, because negative gene flow has no meaning (Hudson et al. 1992). Combosch and Vollmer (2011) found no correlation between genetic differentiation and distance in Pocillopora damicornis over the limited geographic extent of their samples, but our analysis of populations from Mexico to Panama of the data of Pinzón and LaJeunesse (2011) shows that such a correlation does exist in Pocillopora type 1 (r = 0.349, p < 0.005). Baums et al. (2012) found a strong isolation by distance trend in Porites lobata in the ETP, including the outer islands. The correlation remains significant when only coastal localities are considered (r = 0.856, 0.01 < p < 0.025). In Epinephelus labriformis, most FST values were negative (and replaced by 0), which resulted in a slight, but still significant correlation with geographic distance (r = 0.051, 0.01 < p < 0.025). Saarman et al. (2010) reported no significant correlation between FST and distance in Hippocampus ingens, which would have been surprising given that sea horses are sedentary and have no larval stage. Our re-analysis of their FST data, however, shows that the expected relationship does, in fact, exist (r = 0.441, 0.01 < p < 0.025). The difference between their analysis and ours is not only that they (presumably) used negative FST values, but also because some of the geographic distances they listed in their Table 4 cannot be correct because they show Peru situated north of Ecuador. Thus, the available data are consistent with a general trend of isolation by distance along the ETP coast. Such a correlation, however, is not necessarily the result of genes dispersing via stepping stones, because populations on either side of a barrier are also more distant from each other than populations on the same side of the barrier. Riginos and Nachman (2001), using partial Mantel tests, found that genetic divergence of populations of Axoclinus nigricaudus in the Sea of Cortez was caused not only by the distance between localities, but also by a genetic break between the upper and central parts of the Gulf of California.
In conclusion, gene flow rates among populations along the ETP coast are high, at least between central Mexico and the Panamanian coast. There is no evident genetic break resulting from the Central American Gap in any of the sampled species. Populations at the northernmost and the southernmost peripheries of the ETP appear to be genetically more isolated, possibly as the result of historical barriers, or possibly due to ecological conditions unfavorable to tropical species. A trend of isolation by distance is evident in corals and fishes.
16.4 General Conclusions and Future Prospects
The compilation of existing data regarding gene flow of coral reef organisms in the ETP suggests that gene flow within this oceanic region is generally high; this is certainly true along the coast, except perhaps for the northernmost and southernmost limits of tropical species ranges. Documented genetic connectivity is also generally high between populations at the outer ETP islands <1000 km offshore and the mainland, and (in some transpacific species) between the residents of islands in the central Pacific and ETP. These generalizations, however, need to be tempered by the realization that all coral reef organisms in which genetic structure has been sampled to date, except for sea horses, possess planktonic larvae. Large genetic differences among closely situated populations of the same morph of the ovoviviparous intertidal isopod Excirolana braziliensis were found in both isozymes (Lessios and Weinberg 1993, 1994) and in mtDNA (Sponer and Lessios 2009). The same may well turn out to be true for coral reef organisms with limited means of dispersal. It would be of interest to obtain data from ascidians, bryozoans, and other organisms with abbreviated larval phases to see how they compare with data from organisms with planktonic larvae. It is also important to sample genetically more species of corals, the ecological engineers responsible for creating the habitats in which other coral reef organisms live. The isolation of the ETP from other oceanic regions, the remote location of its outer islands, and the simple spatial arrangement of its coastal populations, can produce interesting contrasts in patterns of gene flow. Such data can address general population genetic theory in addition to producing information regarding the natural history of the organisms in this ocean.
References
Abbott DP (1966) Factors influencing the zoogeographic affinities of the Galápagos. In: Bowman RI (ed) the Galápagos. Univ California Press, Berkeley, pp 108–112
Baird AH, Guest JR, Willis BL (2009) Systematic and biogeographical patterns in the reproductive biology of scleractinian corals. Annu Rev Ecol Evol S 40:551–571
Baums IB, Boulay JN, Polato NR, Hellberg ME (2012) No gene flow across the Eastern Pacific Barrier in the reef-building coral Porites lobata. Mol Ecol 21:5418–5433
Bernardi G, Alva-Campbell YR, Gasparini JL, Floeter SR (2008) Molecular ecology, speciation, and evolution of the reef fish genus Anisotremus. Mol Phylogenet Evol 48:929–935
Bowman RI (1966) The Galápagos. Univ California Press, Berkeley, California, p 318
Briggs JC (1974) Marine zoogeography. McGraw-Hill, New York, p 475
Briggs JC, Bowen BW (2012) A realignment of marine biogeographic provinces with particular reference to fish distributions. J Biogeogr 39:12–30
Case TJ, Taper ML (2000) Interspecific competition, environmental gradients, gene flow, and the coevolution of species’ borders. Am Nat 155:583–605
Coates AG, Obando JA (1996) The geologic evolution of the Central American Isthmus. In: Jackson JBC, Coates AG, Budd A (eds) Evolution and environment in tropical America. Univ Chicago Press, Chicago, pp 21–56
Collins LS (1996) Environmental changes in Caribbean shallow waters relative to the closing tropical American seaway. In: Jackson JBC, Budd AF, Coates AG (eds) Evolution and environment in tropical America. Univ Chicago Press, Chicago, pp 130–167
Combosch DJ, Guzmán HM, Schuhmacher H, Vollmer SV (2008) Interspecific hybridization and restricted trans-Pacific gene flow in the Tropical Eastern Pacific Pocillopora. Mol Ecol 17:1304–1312
Combosch DJ, Vollmer SV (2011) Population genetics of an ecosystem-defining reef coral Pocillopora damicornis in the Tropical Eastern Pacific. PLoS ONE 6(8):e21200. doi:10.1371/journal.pone.0021200
Craig MT, Hastings PA, Pondella DJ, Robertson DR, Rosales-Casian JA (2006) Phylogeography of the flag cabrilla Epinephelus labriformis (Serranidae): implications for the biogeography of the Tropical Eastern Pacific and the early stages of speciation in a marine shore fish. J Biogeogr 33:969–979
Craig MT, Eble JA, Bowen BW, Robertson DR (2007) High genetic connectivity across the Indian and Pacific Oceans in the reef fish Myripristis berndti (Holocentridae). Mar Ecol Prog Ser 334:245–254
Dana TF (1975) Development of contemporary eastern Pacific coral reefs. Mar Biol 33:355–374
Dawson MN, Grosberg RK, Stuart YE, Sanford E (2010) Population genetic analysis of a recent range expansion: mechanisms regulating the poleward range limit in the volcano barnacle Tetraclita rubescens. Mol Ecol 19:1585–1605
Duda TF, Lessios HA (2009) Connectivity of populations within and between major biogeographic regions of the tropical Pacific in Conus ebraeus, a widespread marine gastropod. Coral Reefs 28:651–659
Duda TF, Terbio M, Chen G, Phillips S, Olenzek AM, Chang D, Morris DW (2012) Patterns of population structure and historical demography of Conus species in the tropical Pacific. Am Malacol Bull 30:175–187
Ekman S (1953) Zoogeography of the sea. Sidgwick and Jackson Ltd, London, p 417
Emerson WK (1978) Mollusks with Indo-Pacific faunal affinities in the eastern Pacific Ocean. Nautilus 92:91–96
Emerson WK (1982) Zoogeographic implications of the occurrence of Indo-Pacific gastropods on the west American continental borderland. West Soc Malacol Ann Rep 1982:13–14
Fell FJ (1974) The echinoids of Easter Island (Rapa Nui). Pac Sci 28:147–158
Fitzpatrick JM, Carlon DB, Lippe C, Robertson DR (2011) The west Pacific diversity hotspot as a source or sink for new species? Population genetic insights from the Indo-Pacific parrotfish Scarus rubroviolaceus. Mol Ecol 20:219–234
Forsman ZH, Barshis DJ, Hunter CL, Toonen RJ (2009) Shape-shifting corals: molecular markers show morphology is evolutionarily plastic in Porites. BMC Evol Biol 9:45. doi:10.1186/1471-2148-9-45
Glynn PW (1974) The impact of Acanthaster on corals and coral reefs in the eastern Pacific. Environ Conserv 1:295–304
Glynn PW, Wellington GM (1983) Corals and coral reefs of the Galápagos Islands. Univ California Press, Berkeley, p 330
Glynn PW, Ault JS (2000) A biogeographic analysis and review of the far eastern Pacific coral reef region. Coral Reefs 19:1–23
Glynn PW, Gassman NJ, Eakin CM, Cortés J, Smith DB, Guzmán HM (1991) Reef coral reproduction in the eastern Pacific: Costa Rica, Panama, and Galápagos Islands (Ecuador).1. Pocilloporidae. Mar Biol 109:355–368
Glynn PW, Veron JEN, Wellington GM (1996) Clipperton Atoll (eastern Pacific): oceanography, geomorphology, reef-building coral ecology and biogeography. Coral Reefs 15:71–99
Glynn PW, Wellington GM, Riegl B, Olson DB, Borneman E, Wieters EA (2007) Diversity and biogeography of the scleractinian coral fauna of Easter Island (Rapa Nui). Pac Sci 61:67–90
Grigg RW, Hey R (1992) Paleoceanography of the tropical eastern Pacific Ocean. Science 255:172–178
Grove JS, Lavenberg RJ (1997) The fishes of the Galápagos Islands. Stanford Univ Press, Stanford, CA, p 871
Guzmán HM, Guevara CA, Breedy O (2004) Distribution, diversity, and conservation of coral reefs and coral communities in the largest marine protected area of Pacific Panama (Coiba Island). Environ Conserv 31:111–121
Haldane JBS (1956) The relation between density regulation and natural selection. Proc Royal Soc London Ser B-Biol Sci 145:306–308
Hastings PA (2000) Biogeography of the Tropical Eastern Pacific: distribution and phylogeny of chaenopsid fishes. Zool J Linn Soc 128:319–335
Haug GH, Tiedemann R (1998) Effect of the formation of the Isthmus of Panama on Atlantic Ocean thermohaline circulation. Nature 393:673–676
Heck KL, McCoy ED (1978) Long-distance dispersal and the reef-building corals of the eastern Pacific. Mar Biol 48:349–356
Hedrick PW (2005) A standardized genetic differentiation measure. Evolution 59:1633–1638
Hellberg ME (2006) No variation and low synonymous substitution rates in coral mtDNA despite high nuclear variation. BMC Evolutionary Biology 6:24. doi:10.1186/1471-2148-6-24
Hellberg ME (2007) Footprints on water: the genetic wake of dispersal among reefs. Coral Reefs 26:463–473
Hellberg ME (2009) Gene flow and isolation among populations of marine animals. Annu Rev Ecol Evol S 40:291–310
Hellberg ME, Burton RS, Neigel JE, Palumbi SR (2002) Genetic assessment of connectivity among marine populations. Bull Mar Sci 70:273–290
Hertlein LG (1963) Contribution to the biogeography of Cocos Island, including a bibliography. Proc Calif Acad Sci 32:219–289
Hey J, Nielsen R (2004) Multilocus methods for estimating population sizes, migration rates and divergence time, with applications to the divergence of Drosophila pseudoobscura and D. persimilis. Genetics 167:747–760
Hudson RR, Slatkin M, Maddison WP (1992) Estimation of levels of gene flow from DNA sequence data. Genetics 132:583–589
James MJ (1991) Galápagos marine invertebrates. Taxonomy, biogeography, and evolution in Darwin’s islands. Plenum, New York, p 474
Jones GP, Milicich MJ, Emslie MJ, Lunow C (1999) Self-recruitment in a coral reef fish population. Nature 402:802–804
Johnson MS, Black R (1984) Pattern beneath the chaos: the effects of recruitment on genetic patchiness in an intertidal limpet. Evolution 38:1371–1383
Keigwin LD (1982) Isotopic paleoceanography of the Caribbean and east Pacific: role of Panama uplift in Late Neogene time. Science 217:350–353
Kessler WS (2006) The circulation of the eastern tropical Pacific: a review. Prog Oceanogr 69:181–217
Ketchum JT, Bonilla HR (2001) Taxonomy and distribution of the hermatypic corals (Scleractinia) of the Revillagigedo Archipelago, Mexico. Rev Biol Trop 49:803–848
Lessios HA (2008) The great American schism: divergence of marine organisms after the rise of the Central American Isthmus. Annu Rev Ecol Evol S 39:63–91
Lessios HA, Weinberg JR (1993) Migration, gene flow and reproductive isolation between and within morphotypes of the isopod Excirolana in two oceans. Heredity 71:561–573
Lessios HA, Weinberg JR (1994) Genetic and morphological divergence among morphotypes of the isopod Excirolana on the two sides of the Isthmus of Panama. Evolution 48:530–548
Lessios HA, Robertson DR (2006) Crossing the impassable: genetic connections in 20 reef fishes across the eastern Pacific barrier. Proc R Soc B-Biol Sci 273:2201–2208
Lessios HA, Kessing BD, Wellington GM, Graybeal A (1996) Indo-Pacific echinoids in the tropical eastern Pacific. Coral Reefs 15:133–142
Lessios HA, Kessing BD, Robertson DR (1998) Massive gene flow across the world’s most potent marine biogeographic barrier. Proc R Soc Lond Ser B 265:583–588
Lessios HA, Kessing BD, Robertson DR, Paulay G (1999) Phylogeography of the pantropical sea urchin Eucidaris in relation to land barriers and ocean currents. Evolution 53:806–817
Lessios HA, Kessing BD, Pearse JS (2001) Population structure and speciation in tropical seas: global phylogeography of the sea urchin Diadema. Evolution 55:955–975
Lessios HA, Kane J, Robertson DR (2003) Phylogeography of the pantropical sea urchin Tripneustes: contrasting patterns of population structure between oceans. Evolution 57:2026–2036
Lessios HA, Lockhart S, Collin R, Sotil G, Sanchez-Jerez P, Zigler KS, Perez AF, Garrido MJ, Geyer LB, Bernardi G, Vacquier VD, Haroun R, Kessing BD (2012) Phylogeography and bindin evolution in Arbacia, a sea urchin genus with an unusual distribution. Mol Ecol 21:130–144
Madsen FJ (1955) A note on the sea star genus Acanthaster. Vidensk Medd Dansk Naturhist Foren Kbh 117:179–192
Mantel N (1967) The detection of disease clustering and the generalized regression approach. Cancer Res 27:209–220
Marko PB, Hart MW (2012) Retrospective coalescent methods and the reconstruction of metapopulation histories in the sea. Evol Ecol 26:291–315
McCartney MA, Keller G, Lessios HA (2000) Dispersal barriers in tropical oceans and speciation of Atlantic and eastern Pacific Echinometra sea urchins. Mol Ecol 9:1391–1400
McCoy ED, Heck KL Jr (1976) Biogeography of corals, sea grasses, and mangroves: an alternative to the center of origin concept. Syst Zool 25:201–210
Montes C, Cardona A, McFadden R, Moron SE, Silva CA, Restrepo-Moreno S, Ramirez DA, Hoyos N, Wilson J, Farris D, Bayona GA, Jaramillo CA, Valencia V, Bryan J, Flores JA (2012) Evidence for middle Eocene and younger land emergence in central Panama: implications for Isthmus closure. Geol Soc Am Bull 124:780–799
Muss A, Robertson DR, Stepien CA, Wirtz P, Bowen BW (2001) Phylogeography of Ophioblennius: the role of ocean currents and geography in reef fish evolution. Evolution 55:561–572
Nishida M, Lucas JS (1988) Genetic differences between geographic populations of the crown-of-thorns starfish throughout the Pacific region. Mar Biol 98:359–368
O’Dea A, Jackson JBC, Fortunato H, Smith JT, D’Croz L, Johnson KG, Todd JA (2007) Environmental change preceded Caribbean extinction by 2 million years. Proc Natl Acad Sci USA 104:5501–5506
Pinzón JH, LaJeunesse TC (2011) Species delimitation of common reef corals in the genus Pocillopora using nucleotide sequence phylogenies, population genetics and symbiosis ecology. Mol Ecol 20:311–325
Pluzhnikov A, Donnelly P (1996) Optimal sequencing strategies for surveying molecular genetic diversity. Genetics 144:1247–1262
Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure using multilocus genotype data. Genetics 155:945–959
Randall JE (1998) Zoogeography of shore fishes of the Indo-Pacific region. Zool Stud 37:227–268
Rehder HA (1980) The marine mollusks of Easter Island (Isla de Pascua) and Sala y Gómez. Smithson Contrib Zool 289:1–167
Reyes-Bonilla H, Barraza JE (2003) Corals and associated marine communities from El Salvador. In: Cortés J (ed) Latin American coral reefs. Elsevier, Amsterdam, pp 351–360
Richmond RH (1990) The effects of the El Niño/Southern Oscillation on the dispersal of corals and other marine organisms. In: Glynn PW (ed) Global ecological consequences of the 1982-83 El Niño-Southern Oscillation. Elsevier, Amsterdam, pp 127–140
Riginos C (2005) Cryptic vicariance in Gulf of California fishes parallels vicariant patterns found in Baja California mammals and reptiles. Evolution 59:2678–2690
Riginos C, Nachman MW (2001) Population subdivision in marine environments: the contributions of biogeography, geographical distance and discontinuous habitat to genetic differentiation in a blennioid fish, Axoclinus nigricaudus. Mol Ecol 10:1439–1453
Robertson DR (1998) Do coral-reef fish faunas have a distinctive taxonomic structure? Coral Reefs 17:179–186
Robertson DR, Allen GR (1996) Zoogeography of the shorefish fauna of Clipperton Atoll. Coral Reefs 15:121–131
Robertson DR, Cramer KL (2009) Shore fishes and biogeographic subdivisions of the Tropical Eastern Pacific. Mar Ecol Prog Ser 380:1–17
Robertson DR, Grove JS, McCosker JE (2004) Tropical transpacific shore fishes. Pac Sci 58:507–565
Rosenblatt RH, Waples RS (1986) A genetic comparison of allopatric populations of shore fish species from the eastern and central Pacific Ocean: dispersal or vicariance? Copeia 1986:275–284
Saarman NP, Louie KD, Hamilton H (2010) Genetic differentiation across eastern Pacific oceanographic barriers in the threatened seahorse Hippocampus ingens. Conserv Genet 11:1989–2000
Sexton JP, McIntyre PJ, Angert AL, Rice KJ (2009) Evolution and ecology of species range limits. Annu Rev Ecol Evol S 40:415–436
Shearer TL, Van Oppen MJH, Romano SL, Worheide G (2002) Slow mitochondrial DNA sequence evolution in the Anthozoa (Cnidaria). Mol Ecol 11:2475–2487
Slatkin M (1995) A measure of population subdivision based on microsatellite allele frequencies. Genetics 139:457–462
Sponer R, Lessios HA (2009) Mitochondrial phylogeography of the intertidal isopod Excirolana braziliensis on the two sides of the Isthmus of Panama. Smith Contr Mar Sci 38:219–228
Stepien CA, Rosenblatt RH, Bargmeyer BA (2001) Phylogeography of the spotted sand bass, Paralabrax maculatofasciatus: divergence of Gulf of California and Pacific Coast populations. Evolution 55:1852–1862
Swearer SE, Caselle JE, Lea DW, Warner RR (1999) Larval retention and recruitment in an island population of a coral-reef fish. Nature 402:799–802
Swearer SE, Shima JS, Hellberg ME, Thorrold SR, Jones GP, Robertson DR, Morgan SG, Selkoe KA, Ruiz GM, Warner RR (2002) Evidence of self-recruitment in demersal marine populations. Bull Mar Sci 70:251–271
Taylor MS, Hellberg ME (2003) Genetic evidence for local retention of pelagic larvae in a Caribbean reef fish. Science 299:107–109
Todd PA (2008) Morphological plasticity in scleractinian corals. Biol Rev 83:315–337
Vermeij GJ (1978) Biogeography and adaptation. Harvard Univ Press, Cambridge, Mass, p 332
Veron JEN (1995) Corals in space and time: the biogeography and evolution of the Scleractinia. Ithaca, Comstock/Cornell, p 321
Vogler C, Benzie J, Lessios H, Barber P, Worheide G (2008) A threat to coral reefs multiplied? Four species of Crown-of-thorns Starfish. Biology Lett 4:696–699
Webb SD (1976) Mammalian faunal dynamics of the great American interchange. Paleobiology 2:220–234
Whitlock MC, McCauley DE (1999) Indirect measures of gene flow and migration: FST≠ 1/(4Nm+ 1). Heredity 82:117–125
Willis BL, van Oppen MJH, Miller DJ, Vollmer SV, Ayre DJ (2006) The role of hybridization in the evolution of reef corals. Annu Rev Ecol Evol S 37:489–517
Wright S (1943) Isolation by distance. Genetics 28:114–138
Wright S (1951) The genetic structure of populations. Ann Eugen 15:323–354
Yakovleva IM, Baird AH, Yamamoto HH, Bhagooli R, Nonaka M, Hidaka M (2009) Algal symbionts increase oxidative damage and death in coral larvae at high temperatures. Mar Ecol Prog Ser 378:105–112
Zigler KS, Lessios HA (2003) Evolution of bindin in the pantropical sea urchin Tripneustes: comparisons to bindin of other genera. Mol Biol Evol 20:220–231
Acknowledgements
We thank G. Bernardi, S. Coppard, L. Geyer, A. Hiller, P.W. Glynn, and C. Riginos for comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Lessios, H.A., Baums, I.B. (2017). Gene Flow in Coral Reef Organisms of the Tropical Eastern Pacific. In: Glynn, P., Manzello, D., Enochs, I. (eds) Coral Reefs of the Eastern Tropical Pacific. Coral Reefs of the World, vol 8. Springer, Dordrecht. https://doi.org/10.1007/978-94-017-7499-4_16
Download citation
DOI: https://doi.org/10.1007/978-94-017-7499-4_16
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-017-7498-7
Online ISBN: 978-94-017-7499-4
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)