Abstract
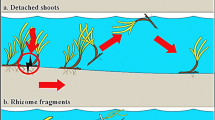
Seagrass colonise new areas via the dispersion of seeds or vegetative fragments. Independent of the manner of colonization, habitat requirements need to be met for the successful establishment of seagrasses. Here we report on the colonization process of Posidonia oceanica in a highly disturbed area: a gas pipeline trench at Capo Feto (SW Sicily, Italy). A trench dredged through a P. oceanica bed was back-filled with rubble added from dump barges leading to the formation of a series of rubble mounds on the seabed. Over time, these mounds became colonised with P. oceanica. I>. In order to understand the pattern of P. oceanica colonization, shoot density was quantified over 3 years (2001–2003) on different mound locations (crests, sides, valleys). Seagrass coalescence was observed only in valleys between mounds where shoot density averaged 133±50 shoots m−2, while values for sides and crests were significantly lower (30.5±14 and 5.8±2.6 shoots m−2, respectively). Although sediment accumulated on both crests and valleys, a significantly thicker sediment layer was recorded in the valleys (9.8±0.4 cm) than on crests (1.1±0.2). Plaster dissolution rate (an indicator of the hydrodynamic regime) tended to decrease from crests to valleys but even in the valleys, the currents were still higher than in the adjacent vegetated control location. This pattern was constant over time and depths. This is the first study to report on P. oceanica vegetative recruitment on artificial rubble after a disturbance event. It appears that the valleys between the rubble mounds are suitable for seagrass recruitment as sediment deposited between the rubble provides the necessary resources for plant settlement and growth. Once the seagrass patches are established, they may start a positive feedback of attenuation of currents, sediment accumulation and seagrass patch expansion.





Similar content being viewed by others
References
Ackerman JD, Okubo A (1983) Reduced mixing in a marine macrophyte canopy. Funct Ecol 70:305–309
Bailey-Brock JH (1979) Sediment trapping by chaetopterid polychaetes on a Hawaiian fringing reef. J Mar Res 37(4):643–656
Balestri E, Piazzi L, Cinelli F (1998) Survival and growth of transplanted and natural seedlings of Posidonia oceanica (L.) Delile in a damaged coastal area. J Exp Mar Biol Ecol 228:209–225
Campbell ML (2003) Recruitment and colonization of vegetative fragments of Posidonia australis and Posidonia coriacea. Aquat Bot 76:175–184
D’Anna G, Badalamenti F, Riggio S (2000) Artificial reefs in North West Sicily: comparisons and conclusion. In: Jensen AC, Collins KJ, Lockwood APM (eds) European artificial reefs in European seas. Kluwer, London, pp 97–112
Dauby P, Bale AJ, Bloomer N, Canon C, Ling RD, Norro A, Robertson JE, Simon A, Theate JM, Watson AJ, Frankignoulle M (1995) Particle fluxes over a Mediterranean seagrass bed: a one year case study Mar Ecol Prog Ser 126(1–3):233–246
Delgado O, Ruiz JM, Perez M, Romero J, Ballestreros E (1999). Effects of fish farming on seagrass (Posidonia oceanica) in a Mediterranean Bay: seagrass decline after organic loading cessation. Oceanol Acta 22(1):109–117
Denslow JS (1985) Disturbance-mediated coexistence of species. In: Pickett STA, White PS (eds) The ecology of natural disturbance and patch dynamics. Academic, London, pp 307–321
Di Carlo G, Badalamenti F, Passalacqua C (2004) The use of reconstructive methods in combination with ‘beyond BACI’ designs: the case study of Capo Feto (SW Sicily, Italy). Rapp Comm Int Mer Médit 37:514
Dierssen H, Zimmerman R, Leathers R, Downes T, Davis C (2003) Ocean colour remote sensing of seagrass and bathymetry in the Bahamas Banks by high-resolution airborne imagery. Limnol Oceanogr 48:444–455
Doty M (1974) Measurements of water movement in reference to benthic algal growth. Bot Mar XIV:32–35
Duarte CM (2002) The future of seagrass meadows. Environ Conserv 29(2):192–206
Duarte CM, Sand-Jensen K (1990) Seagrass colonization: patch formation and patch growth in Cymodocea nodosa. Mar Ecol Prog Ser 65:193–200
Fonseca MS, Zieman JC, Thayer GW, Fisher JS (1983) The role of current velocity in structuring eelgrass (Zostera marina L.) meadows. Estuar Coast Shelf Sci 17:367–380
Francour F (1997) Fish assemblages of Posidonia oceanica beds at Port-Cros (France, NW Mediterranean): assessment of composition and long-term fluctuations by visual census. Mar Ecol 18(2):157–173
Gacia E, Duarte C (2001) Sediment retention by a Mediterranean Posidonia oceanica meadow: the balance between deposition and resuspension. Estuar Coast Shelf Sci 52:505–514
Gacia E, Granata TC, Duarte CM (1999) An approach to measurement of particle flux and sediment retention within seagrass (Posidonia oceanica) meadows. Aquat Bot 65(1–4):255–268
Gambi MC, Buia MC, Casola E, Scardi M (1989) Estimates of water movement in Posidonia oceanica beds: a first approach. In: Boudouresque CF, Meinesz A, Fresi E, Gravez V (eds) Second international workshop on Posidonia beds. GIS Posidonie Publ, Marseilles, pp 101–112
Gambi MC, Nowell AR, Jumars PA (1990) Flume observations on flow dynamics in Zostera marina (eelgrass) beds. Mar Ecol Prog Ser 61:159–169
Granata TC, Serra T, Colomer J, Casamitjana X, Duarte CM, Gacia E (2001) Flow and particle distributions in a nearshore seagrass meadow before and after a storm. Mar Ecol Prog Ser 218:95–106
Green EP, Short FT (2003) World Atlas of Seagrasses. University of California Press, Berkeley
Hemminga M, Duarte CM (2000) Seagrass ecology. Cambridge University Press, Cambridge
Kenworthy WJ, Fonseca MS, Whitfield PE, Hammerstrom KK (2002) Analysis of seagrass recovery in experimental excavations and propeller-scar disturbances in the Florida Keys National Marine Sanctuary. J Coast Res 37:75–85
van Keulen M, Borowitzka MA (2000) Comparison of water velocity profiles through morphologically dissimilar seagrasses. Biol Mar Medit 7:143–146
Koch EW (2001) Beyond light: physical, geological, and geochemical parameters as possible submersed aquatic vegetation habitat requirements. Estuaries 24(1):1–17
Koch EW, Verduin JJ (2001) Measurement of physical parameters in seagrass habitats. In: Short FT, Coles RG (ed) Global seagrass research methods. Elsevier, Amsterdam, pp 325–344
Komatsu T, Kawai H (1992) Measurements of time-averaged intensity of water motion with plaster balls. J Oceanogr 48:535–365
Long WJL, Thom RM (2001) Improving seagrass habitat quality. In: Short FT, Coles RG (ed) Global seagrass research methods. Elsevier, Amsterdam, pp 407–423
Marbà N, Duarte CM (1998) Rhizome elongation and seagrass clonal growth. Mar Ecol Prog Ser 174:269–280
Marbà N, Cebrián J, Enríquez S, Duarte CM (1996) Growth patterns of Western Mediterranean seagrasses: species-specific responses to seasonal forcing. Mar Ecol Prog Ser 133:203–215
Meinesz A, Lefèvre JR (1984) Régénération d’un herbier de Posidonia oceanica quarante années aprés sa destruction par une bombe dans la rade de Villefranche (Alpes-Maritimes, France). In: Boudouresque CF, Jeudy de Grissac A, Olivier J (eds) International Workshop Posidonia oceanica Beds. Gis Posidonie, France, pp 39–44
Meinesz A, Molenaar H, Caye G (1992) Transplantation de phanérogames marines en Méditerranée. Comm In Expl Sc Mer Médit XXXIII:1–8
Molenaar H, Meinesz A (1995) Vegetative reproduction: survival and development of transplanted cuttings according to different spacings, arrangements and substrates. Bot Mar 38:313–322
Moriarty DJW, Boon PI (1989) Interactions of seagrasses with sediment and water. In: Larkum AWD, McComb AJ, Shepherd SA (eds) Biology of seagrasses: a treatise on the biology of seagrasses with special reference to the Australian region. Elsevier, New York, pp 500–535
Muus BJ (1968) A field method for measuring “exposure” by mean of plaster balls: a preliminary account. Sarsia 34:61–68
Pergent-Martini C, Pasqualini V (2000) Seagrass population dynamics before and after the setting up of a wastewater treatment plant. Biol Mar Medit 7(2):405–408
Piazzi L, Balestri E (1997) Osservazioni sulla fioritura e fruttificazione di trapianti di Posidonia oceanica (L.) Delile. Biol Mar Medit 4:429–430
Porter ET, Sanford LP, Suttles SE (2000) Gypsum dissolution is not a universal integrator of “water motion”. Limnol Oceanogr 45:145–158
Rasheed MA (1999) Recovery of experimentally created gaps within a tropical Zostera capricorni (Aschers.) seagrass meadow, Queensland Australia. J Exp Mar Biol Ecol 235:183–200
Rasheed MA (2004) Recovery and succession in a multi-species tropical seagrass meadow following experimental disturbance: the role of sexual and asexual reproduction. J Exp Mar Biol Ecol 310:13– 45
Riggio S (1995) Le barriere artificiali e l’uso conservativo della fascia costiera: risultati dei “reefs” della Sicilia N/O. Biol Mar Medit 2(1):129–164
Ruiz JM, Perez M, Romero J (2001) Effects of fish farm loadings on seagrass (Posidonia oceanica) distribution, growth and photosynthesis. Mar Poll Bull 42 (9):749–760
Scoffin TP (1970) The trapping and binding of subtidal carbonate sediments by marine vegetation in Bimini Lagoon, Bahamas. J Sediment Petrol 40:249–273
Snedecor GW, Cochran WG (1989) Statistical methods, 8th edn. Iowa State University Press, Ames
Thayer GW, Kenworthy WJ, Fonseca MS (1984). The ecology of eelgrass meadows of the Atlantic coast: a community profile, US Fish and Wildlife Service, FWS/OBS-84/ 02
Thompson TL, Glenn EP (1994) Plaster standards to measure water motion. Limnol Oceanogr 39:1768–1779
Underwood AJ (1981) Techniques of analysis of variance in experimental marine biology and ecology. Oceanograph Mar Biol Annu Rev 19:513–605
Underwood AJ (1997) Experiments in ecology: their logical design and interpretation using analysis of variance. Cambridge University Press, Cambridge
Venturi M, Petti M, Drago M (1998) Wave crest properties and non-linearities of storm waves measured at three sites in the Mediterranean Sea. Proc Int Offshore Polar Eng Conf 3(8):129–136
Vidondo B, Duarte CM, Middelboe AL, Stefansen K, Lützen T, Nielsen SL (1997) Dynamics of landscape mosaic: size and age distribution, growth and demography of seagrass Cymodocea nodosa patches. Mar Ecol Prog Ser 158:131–138
Zieman JC (1972) Origin of circular beds of Thalassia (Spermatophyta: Hydrocharitaceae) in South Biscayne Bay, Florida, and their relationship to mangrove hammocks. Bull Mar Sci 22(3):559–574
Acknowledgements
The authors kindly thank TMPC (Trans-Mediterranean Pipeline Company Ltd) for the co-operation to realise this study. We also wish to acknowledge G Albano (Mariconsult), M Gristina, T Vega Fernandez, M Bascone for help during field work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by R. Cattaneo-Vietti, Genova
Rights and permissions
About this article
Cite this article
Di Carlo, G., Badalamenti, F., Jensen, A.C. et al. Colonisation process of vegetative fragments of Posidonia oceanica (L.) Delile on rubble mounds. Marine Biology 147, 1261–1270 (2005). https://doi.org/10.1007/s00227-005-0035-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-005-0035-0