Abstract
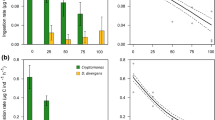
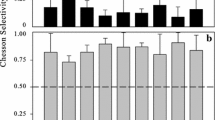
In this work we studied the trophic ecology and feeding impact of the cladoceran Penilia avirostris and the cyclopoid copepod Oithona nana, the two dominant zooplankters in the summer communities of the coastal NW Mediterranean, on the naturally occurring microbial communities. In order to ascertain carbon surplus for growth and reproduction and the contribution to carbon and nitrogen recycling of these two species, we also determined their basal metabolism and excretion rates. The experiments conducted during summers 2002, 2003, and 2004 indicate that P. avirostris grazed mostly upon small flagellates, dinoflagellates, and diatoms, whereas O. nana had a narrower prey range, selecting motile organisms such as ciliates and occasionally dinoflagellates. The grazing impact of both species accounted, on average, for <10% of the standing stock of the microbial groups considered. In spite of the oligotrophic conditions, the feeding activity of P. avirostris is in general sufficient to compensate basal metabolism and allows a surplus for growth and reproduction. This was not the case for O. nana, its daily rations being often lower than the carbon basal demands. Regarding excretion rates, both species presented different N:P excretion ratios, the ones of O. nana falling within values typical for copepods, whereas the absence of detectable phosphorus excretion by P. avirostris implied an unbalance recycling with respect to typical Redfield ratio composition of marine seston.



Similar content being viewed by others
References
Alcaraz M (1970) Ciclo anual de los cladóceros en aguas de Castellón (Mediterráneo occidental). Inv Pesq 34:281–290
Alcaraz M (1981) Ciclo anual de los cladóceros y ostrácodos planctónicos en la plataforma continental de Vizcaya (Punta Endata). Inv Pesq 45:3–16
Andersen T, Elser JJ, Hessen DO (2004) Stoichiometry and population dynamics. Ecol Lett 7:884–900
Atienza D, Saiz E, Calbet A (2006) Feeding ecology of the marine cladoceran Penilia avirostris. Natural diets, daily ration and prey selectivity. Mar Ecol Prog Ser (in press)
Båmstedt U, Gifford DJ, Irigoien X, Atkinson A, Roman M, Harris RP (2000) Feeding. In: Wiebe PH, Lenz J, Skjoldal HR, Huntley M (eds) ICES Zooplankton Methodology Manual. Academic, UK, pp 297–399
Berggren U, Hansen B, Kiorboe T (1988) Food size spectra, ingestion and growth of the copepod Acartia tonsa during development: implications for determination of copepod production. Mar Biol 99:341–352
Børsheim KY, Bratbak G (1987) Cell volume to cell carbon conversion factors for a bacterivorous Monas sp. enriched from seawater. Mar Ecol Prog Ser 36:171–175
Broglio E, Saiz E, Calbet A, Trepat I, Alcaraz M (2004) Trophic impact and prey selection by crustacean zooplankton on the microbial communities of an oligotrophic coastal area (NW Mediterranean Sea). Aquat Microb Ecol 35:65–78
Calbet A, Saiz E (2005) The ciliate–copepod link in marine ecosystems. Aquat Microb Ecol 38:157–167
Calbet A, Landry MR, Scheinberg RD (2000) Copepod grazing in a subtropical bay: species-specific responses to a midsummer increase in nanoplankton standing stock. Mar Ecol Prog Ser 193:75–84
Calbet A, Garrido S, Saiz E, Alcaraz M, Duarte M (2001) Annual zooplankton succession in coastal NW Mediterranean waters: the importance of the smaller size fractions. J Plankton Res 23:319–331
Carrillo P, Reche I, Cruz-Pizarro L (1996) Intraspecific stoichiometric variability and the ratio of nitrogen to phosphorus resupplied by zooplankton. Freshw Biol 36:363–374
Castellani C, Robinson C, Smith T, Lampitt RS (2005) Temperature affects respiration rate of Oithona similis. Mar Ecol Prog Ser 285:129–135
Christou ED, Moraitou-Apostopoulou M (1995) Metabolism and feeding of mesozooplankton in the Eastern Mediterranean (Hellenic coastal waters). Mar Ecol Prog Ser 126:39–48
Debs CA (1984) Carbon and nitrogen budget of the calanoid copepod Temora stylifera: effect of concentration and composition of food. Mar Ecol Prog Ser 15:213–223
Egloff DA, Fofonoff PW, Onbé T (1997) Reproductive biology of marine cladocerans. Adv Mar Biol 31:79–168
Elser JJ, Urabe J (1999) The stoichiometry of consumer-driven nutrient cycling: theory, observations, and consequences. Ecology 80:735–751
Elser JJ, Elser MM, MacKay NA, Carpenter SR (1988) Zooplankton-mediated transitions between N- and P- limited algal growth. Limnol Oceanogr 33:1–14
Elser JJ, Dobberfuhl DR, MacKay NA, Schampel JH (1996) Organism size, life history, and N:P stoichiometry: toward a unified view of cellular and ecosystem processes. BioScience 46:674–684
Elser JJ, Sterner RW, Gorokhova E, Fagan WF, Markow TA, Cotner JB, Harrison JF, Hobbie SE, Odell GM, Weider LJ (2000) Biological stoichiometry from genes to ecosystems. Ecol Lett 3:540–550
Frangoulis C, Christou ED, Hecq JH (2005) Comparison of marine copepod outfluxes: nature, rate, fate and role in the carbon and nitrogen cycles. Adv Mar Biol 47:254–309
Frost BW (1972) Effects of size and concentration of food particles on the feeding behavior of the marine planktonic copepod Calanus pacificus. Limnol Oceanogr 17:805–815
Gabriel W, Thomas B (1988) Vertical migration of zooplankton as an evolutionarily stable strategy. Am Nat 132:199–216
Gaudy R, Boucher J (1983) Relation between respiration, excretion (ammonia and inorganic phosphorus) and activity of amylase and trypsin in different species of pelagic copepods from an Indian Ocean equatorial area. Mar Biol 75:37–45
Gaudy R, Cervetto G, Pagano M (2000) Comparison of the metabolism of Acartia clausi and A. tonsa: influence of temperature and salinity. J Exp Mar Biol Ecol 247:51–65
Gismervik I (1997) Stoichiometry of some marine plankton crustaceans. J Plankton Res 19(2):279–285
González HE, Smetacek V (1994) The possible role of the cyclopoid copepod Oithona in retarding vertical flux of zooplankton faecal material. Mar Ecol Prog Ser 113:233–246
Gore MA (1980) Feeding experiments on Penilia avirostris Dana (Cladocera: Crustacea). J Exp Mar Biol Ecol 44:253–260
Gulati RD, Perez Martinez C, Siewertsen K (1995) Zooplankton as a compound mineralizing and synthesizing system: phosphorus excretion. Hydrobiology 315:25–37
Hansen HP, Koroleff F (1999) Determination of nutrients. In: Grasshoff K, Kremling K, Ehrhardt M (eds) Methods of seawater analysis. Wiley-VCH, New York, 159–228
Hansen PJ, Bjørnsen PK, Hansen BW (1997) Zooplankton grazing and growth: scaling within the 2-2000-μm body size range. Limnol Oceanogr 42:687–704
Harris RP (1988) Interactions between diel vertical migratory behavior of marine zooplankton and the subsurface chlorophyll maximum. Bull Mar Sci 43(3):663–674
Hiromi J (1994) Further studies on respiration of the small planktonic copepod Oithona davisae with special reference to the effect of feeding. Bull Col Agric Vet Med Nihon Univ 51:149–153
Hiromi J, Ichihashi O (1995) Influence of starvation length on the phosphate excretion rate of small sized copepod Oithona davisae. Bull Coll Agric Vet Med Nihon Univ 52:119–121
Hiromi J, Nagata T, Kadota S (1988) Respiration of the small planktonic copepod Oithona davisae at different temperatures. Bull Plankton Soc Jpn 35:143–148
Hopcroft RR, Roff JC, Lombard D (1998) Production of tropical copepods in Kingston Harbour, Jamaica: the importance of small species. Mar Biol 130:593–604
Ikeda T, Kanno Y, Ozaki K, Shinada A (2001) Metabolic rates of epipelagic marine copepods as a function of body mass and temperature. Mar Biol 139:587–596
Katechakis A, Stibor H, Sommer U, Hansen T (2002) Changes in the phytoplankton community and microbial food web of Blanes Bay (Catalan Sea, NW Mediterranean) under prolonged grazing pressure by doliolids (Tunicata), cladocerans or copepods (Crustacea). Mar Ecol Prog Ser 234:55–69
Katechakis A, Stibor H, Sommer U, Hansen T (2004) Feeding selectivities and food niche separation of Acartia clausi, Penilia avirostris (Crustacea) and Doliolum denticulatum (Thaliacea) in Blanes Bay (Catalan Sea, NW Mediterranean). J Plankton Res 26:589–603
Kim WC, Lai-Chun C, Quingchao C (1994) Ecology of the marine cladoceranPenilia avirostris Dana in Tolo Harbour, Hong Kong. Acta Oceanol Sin 13:117–127
Lampitt RS, Gamble JC (1982) Diet and respiration of the small planktonic marine copepod Oithona nana. Mar Biol 66:185–190
Lipej L, Mozetic P, Turk V, Malej A (1997) The trophic role of the marine cladoceran Penilia avirostris in the Gulf of Trieste. Hydrobiology 360:197–203
Lonsdale DJ, Caron DA, Dennett MR, Schaffner R (2000) Predation by Oithona spp. on protozooplankton in the Ross Sea, Antarctica. Deep Sea Res II 47:3273–3283
Macedo CF, Pinto-Coelho M (2000) Diel variations in respiration, excretion rates, and nutritional status of zooplankton from the Pampulha reservoir, Belo Horizonte, MG. J Exp Zool 286:671–682
Mayzaud P, Conover RJ (1988) O:N atomic ratio as a tool to describe zooplankton metabolism. Mar Ecol Prog Ser 45:289–302
Mayzaud P, Razouls S, Errhif A, Tirelli V, Labat JP (2002) Feeding, respiration and egg production rates of copepods during austral spring in the Indian sector of the Antarctic Ocean: role of the zooplankton community in carbon transformation. Deep Sea Res I 49:1027–1048
Miller CA, Landry MR (1984) Ingestion-independent rates of ammonium excretion by the copepod Calanus pacificus. Mar Biol 78:265–270
Morán XAG, Estrada M, Gasol JM, Pedrós-Alió C (2002) Dissolved primary production and the strength of phytoplankton-bacterioplankton coupling in contrasting marine regions. Microb Ecol 44:217–223
Nakamura Y, Turner JT (1997) Predation and respiration by the small cyclopoid copepod Oithona similis: how important is feeding on ciliates and heterotrophic flagellates? J Plankton Res 19:1275–1288
Norland S (1993) The relationship between biomass and volume of bacteria. In: Kemp PF, Sherr BF, Sherr EB, Cole JJ (eds) Handbook of methods in aquatic microbial ecology. Lewis Publisher, Florida, pp 303–307
Olsen Y, Jensen A, Reinertsen H, Børshein KY, Heldal M, Langeland A (1986) Dependence of the rate of release of phosphorus by zooplankton on the P:C ratio in the food supply, as calculated by a recycling model. Limnol Oceanogr 31:34–44
Onbé T (1985) Seasonal fluctuations in the abundance of populations of marine cladocerans and their resting eggs in the Inland Sea of Japan. Mar Biol 87:83–88
Onbé T, Ikeda T (1995) Marine cladocerans in Toyama Bay, southern Japan Sea: seasonal occurrence and day–night vertical distributions. J Plankton Res 17(3):595–609
Paffenhöfer GA (1983) On the ecology of marine cyclopoid copepods (Crustacea, Copepoda). J Plankton Res 15:37–55
Paffenhöfer GA, Gardner WS (1984) Ammonium release by juveniles and adult females of the subtropical marine copepod Eucalanus pileatus. J Plankton Res 6:505–513
Paffenhöfer GA, Orcutt JD (1986) Feeding, growth and food conversion of the marine cladoceran Penilia avirostris. J Plankton Res 8:741–754
Parson TR, Maita Y, Lalli CM (1984) A manual of chemical and biological methods for seawater analysis. Pergamon Press, New York, 173 pp
Pavlova EG (1967) Food requirements of the Black Sea cladoceran Penilia avirostris Dana, and how they are met. Fisheries Research Board of Canada Translation Series 908, p 31
Pérez-Martínez C, Gulati RD (1999) Species-specific N and P release rates in Daphnia. Hydrobiology 391:147–155
Picard V, Lair N (2000) The influence of autotrophic and heterotrophic foods on the demography of Daphnia longispina under starved, semi-natural and enriched conditions. J Plankton Res 22:1925–1944
Porter KG, Feig YS (1980) The use of DAPI for identifying and counting aquatic microflora. Limnol Oceanogr 25:943–948
Saiz E, Calbet A (2006) Scaling of feeding in marine calanoid copepods. Limnol Oceanogr (submitted)
Sterner RW (1990) The ratio of nitrogen to phosphorus resupplied by herbivores: zooplankton and the algal competitive arena. Am Nat 136:209–229
Sterner RW, Elser JJ (2002) Ecological stoichiometry: the biology of elements from molecules to the biosphere. Princetown University Press, Princetown
Sterner RW, Elser JJ, Hessen DO (1992) Stoichiometric relationships among producers, consumers and nutrient cycling in pelagic ecosystems. Biogeochemistry 17:49–67
Svensen C, Kiorboe T (2000) Remote prey detection in Oithona similis: hydromechanical vs. chemical cues. J Plankton Res 22:1155–1166
Turner JT, Tester PA, Ferguson RL (1988) The marine cladoceran Penilia avirostris and the “microbial loop” of pelagic food webs. Limnol Oceanogr 33:245–255
Uye S (1982) Length–weight relationships of important zooplankton from the Inland Sea of Japan. J Oceanogr Soc Jpn 38:149–158
Vaqué D, Blough HA, Duarte CM (1997) Dynamics of ciliate abundance, biomass and community composition in an oligotrophic coastal environment (NW Mediterranean). Aquat Microb Ecol 12:71–83
Verity PG, Smetacek V (1996) Organism life cycles, predation, and the structure of marine pelagic ecosystems. Mar Ecol Prog Ser 130:277–293
Walve J, Larsson UL (1999) Carbon, nitrogen and phosphorus stoichiometry of crustacean zooplankton in the Baltic Sea: implications for nutrient recycling. J Plankton Res 21:2309–2321
Waterbury JB, Watson SW, Valois FW, Franks DG (1986) Biological and ecological characterization of the marine unicellular cyanobacterium Synechococcus. Can Bull Fish Aquat Sci 214:71–1201
Wiltshire KH, Lampert W (1999) Urea excretion by Daphnia: a colony-inducing factor in Scenedesmus? Limnol Oceanogr 44:1894–1903
Wong CK, Chan ALC, Tang KW (1992) Natural ingestion rates and grazing of the marine cladoceran Penilia avirostris Dana in Tolo Harbour, Hong Kong. J Plankton Res 14:1757–1765
Zeldis J, James MR, Grieve J, Richards L (2002) Omnivory by copepods in the New Zealand subtropical frontal zone. J Plankton Res 24:9–23
Zöllner E, Santer B, Boersma M, Hoppe HG, Jürgens K (2003) Cascading predation effects of Daphnia and copepods on microbial food web components. Freshw Biol 48:2174–219
Acknowledgements
This work was supported by a PhD fellowship from the Spanish Government to D.A., by the Spanish CICYT projects REN2001-1693 to E.S., and CTM2004-02575/MAR and Program Ramón y Cajal from the Ministry of Education and Science of Spain to A.C. We also want to thank the help of the Captain and crew of the Harbour of Masnou, and Pepito and Ramón who kindly provided facilities and access to the sea. Finally, we want to thank Belén Aguilera for her inestimable help counting the samples, and four anonymous reviewers for their constructive comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S.A. Poulet, Roscoff
Rights and permissions
About this article
Cite this article
Atienza, D., Calbet, A., Saiz, E. et al. Trophic impact, metabolism, and biogeochemical role of the marine cladoceran Penilia avirostris and the co-dominant copepod Oithona nana in NW Mediterranean coastal waters. Mar Biol 150, 221–235 (2006). https://doi.org/10.1007/s00227-006-0351-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-006-0351-z