Abstract
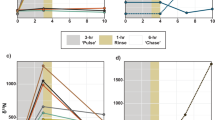
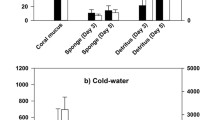
Mucus released by scleractinian corals can act as an important energy and nutrient carrier in coral reef ecosystems, and a distinct isotopic signature would allow following the fate of this material. This study investigates the natural C and N stable isotopic signatures of mucus released by four scleractinian coral genera (Acropora, Fungia, Pocillopora and Stylophora) in comparison with those of suspended particulate organic matter (POM) in seawater of a Northern Red Sea fringing coral reef near Aqaba, Jordan. The natural δ13C and δ15N signatures of coral mucus differed significantly from seawater POM for the majority of seasonal comparisons, but were inappropriate for explicit tracing of mucus in the coral reef food web. Thus, a labeling technique using stable isotope tracers (13C and 15N) was developed that produced δ13C values of up to 122 ± 5‰ (mean ± SE) and δ15N of up to 2,100 ± 151‰ in mucus exuded by Fungia corals. 13C and 15N-enriched compounds were rapidly (within 3 h) and light-dependently transferred from the endosymbiotic zooxanthellae to the mucus-producing coral host. The traceability of 15N-labeled mucus was examined by evaluating its uptake and potential utilization by epizoic acoelomorph Waminoa worms naturally occurring on a range of scleractinian coral taxa. This tracer experiment resulted in uptake of coral mucus by the coral-associated acoelomorphs and further demonstrated the possibility to trace stable isotope-labeled coral mucus by revealing a new trophic pathway in coral reef ecosystems.



Similar content being viewed by others
References
Barneah O, Brickner I, Hooge M, Weis VM, LaJeunesse TC, Benayahu Y (2007) Three party symbiosis: acoelomorph worms, corals and unicellular algal symbionts in Eilat (Red Sea). Mar Biol 151:1215–1223
Barnes R, Hughes R (1999) An introduction to marine ecology. Blackwell Scientific Publications, Oxford
Benson A, Muscatine L (1974) Wax in coral mucus—energy transfer from corals to reef fishes. Limnol Oceanogr 19:810–814
Black CC, Burris JE (1983) Diurnal carbon-14 partitioning between zooxanthellae and the coral animal tissue of intact Seriatopora hystrix colonies. Mar Biol 75:117–120
Boschker HTS, Nold SC, Wellsbury P, Bos D, deGraaf W, Pel R, Parkes RJ, Cappenberg TE (1998) Direct linking of microbial populations to specific biogeochemical processes by 13C-labeling of biomarkers. Nature 392:801–805
Boschker HTS, deBrouwer JFC, Cappenberg TE (1999) The contribution of macrophyte derived organic matter in microbial biomass in salt marsh sediments: stable carbon-isotope analysis of microbial biomarkers. Limnol Oceanogr 44:309–319
Coffroth MA (1984) Ingestion and incorporation of coral mucus aggregates by a Gorgonian soft coral. Mar Ecol Prog Ser 17:193–199
Coffroth MA (1990) Mucous sheet formation on poritid corals—an evaluation of coral mucus as a nutrient source on reefs. Mar Biol 105:39–49
Coplen TB (1994) Reporting of stable hydrogen, carbon, and oxygen isotopic abundances. Pure Appl Chem 66:273–276
Crossland C, Barnes D, Borowitzka M (1980) Diurnal lipid and mucus production in the staghorn coral Acropora acuminata. Mar Biol 60:81–90
Davies PS (1984) The role of zooxanthellae in the nutritional energy requirements of Pocillopora eydouxi. Coral Reefs 2:181–186
DeNiro MJ, Epstein S (1981) Influence of diet on the distribution of nitrogen isotopes in animals. Geochim Cosmochim Acta 45:341–351
Drollet JH, Glaziou P, Martin PMV (1993) A study of mucus from the solitary coral Fungia fungites (Scleractinia: Fungiidae) in relation to photobiological UV adaptation. Mar Biol 115:263–266
Ducklow HW, Mitchell R (1979a) Composition of mucus released by coral reef coelenterates. Limnol Oceanogr 24:706–714
Ducklow H, Mitchell R (1979b) Bacterial populations and adaptations in the mucus layers on living corals. Limnol Oceanogr 24:715–725
Ferrier-Pagès C, Leclercq N, Jaubert J, Pelegri SP (2000) Enhancement of pico- and nanoplankton growth by coral exudates. Aquat Microb Ecol 21:203–209
Fry B (2006) Stable isotope ecology. Springer, Berlin, p 308, ISBN 978–0–387–30513–4
Fry B, Sherr E (1984) δ13C measurements as indicators of carbon flow in marine and freshwater ecosystems. Contrib Mar Sci 27:13–47
Fry B, Mumford PL, Robblee MB (1999) Stable isotope studies of pink shrimp (Farfantepenaeus duorarum Burkenroad) migrations on the southwestern Florida shelf. Bull Mar Sci 65:419–430
Glynn PW (1993) Coral reef bleaching-ecological perspectives. Coral Reefs 12:1–17
Goreau TF, Goreau NI, Goreau TJ (1979) Corals and coral reefs. Sci Am 241:124–135
Gottfried M, Roman MR (1983) Ingestion and incorporation of coral mucus detritus by reef zooplankton. Mar Biol 72:211–218
Grover R, Maguer JF, Allemand D, Ferrier-Pages C (2003) Nitrate uptake in the scleractinian coral Stylophora pistillata. Limnol Oceangr 48:2266–2274
Haapkylä J, Seymour AS, Barneah O, Brickner I, Hennige S, Suggett D, Smith D (2009) Association of Waminoa sp. (Acoela) with corals in the Wakatobi Marine Park, South-East Sulawesi, Indonesia. Mar Biol 156:1021–1027
Heip CHR, Goosen NK, Herman PMJ, Kromkamp J, Middelburg JJ, Soetart K (1995) Production and consumption of biological particles in temperate tidal estuaries. Oceanogr Mar Biol Annu Rev 33:1–150
Herman PMJ, Middelburg JJ, Widdows J, Lucas CH, Heip CHR (2000) Stable isotopes as trophic tracers: combining field sampling and manipulative labeling of food resources for macrobenthos. Mar Ecol Prog Ser 204:79–92
Hoegh-Guldberg O (1999) Climate change, coral bleaching and the future of the world’s coral reefs. Mar Freshw Res 50:839–866
Hooge MD, Haye PA, Tyler S, Litvaitis MK, KornWeld I (2002) Molecular systematics of the Acoela (Acoelomorpha, Platyhelminthes) and its concordance with morphology. Mol Phylogenet Evol 24:333–342
Huettel M, Wild C, Gonelli S (2006) Mucus trap in coral reefs: formation and temporal evolution of particle aggregates caused by coral mucus. Mar Ecol Prog Ser 307:69–84
Johannes R (1967) Ecology of organic aggregates in the vicinity of a coral reef. Limnol Oceanogr 12:189–195
Krupp DA (1984) Mucus production by corals exposed during an extreme low tide. Pac Sci 38:1–11
Lesser MP, Mazel CH, Gorbunov MY, Falkowski PG (2004) Discovery of symbiotic nitrogen-fixing cyanobacteria in corals. Science 305:997–1000
Loya Y (1976) Recolonization of Red Sea corals affected by natural catastrophes and man-made perturbations. Ecology 57:278–289
Mariotti A (1983) Atmospheric nitrogen is a reliable standard for natural 15N abundance measurements. Nature 303:685–687
Marshall M (1968) Observations on organic aggregates in the vicinity of coral reefs. Mar Biol 2:50–55
Marshall AT, Wright OP (1993) Confocal laser scanning light microscopy of the extra-thecal epithelia of undecalcified scleractinian corals. Cell Tissue Res 272:533–543
McCoy AM, Balzer I (2002) Algal symbiosis in flatworms. In: Seckbach J (ed) Symbiosis: mechanisms and model systems. Kluwer, Dordrecht, pp 561–574
Meikle P, Richards G, Yellowlees D (1988) Structural investigations on the mucus from 6 species of coral. Mar Biol 99:187–193
Middelburg JJ, Barranguet C, Boschker HTS, Herman MJ, Moens T, Heip CHR (2000) The fate of intertidal microphytobenthos carbon: an in situ 13C-labeling study. Limnol Oceanogr 45:1224–1234
Minagawa M, Wada E (1984) Stepwise enrichment of 15N along food chains. Further evidence and the relation between δ15N and animal age. Geochim Cosmochim Acta 48:1135–1140
Muscatine L, McCloskey LR, Marian RE (1981) Estimating the daily contribution of carbon from zooxanthellae to coral animal respiration. Limnol Oceanogr 26:601–611
Muscatine L, Falkowski P, Porter J, Dubinsky Z (1984) Fate of photosynthetically-fixed carbon in light and shade-adapted colonies of the symbiotic coral Stylophora pistillata. P Roy Soc B Biol Sci 222:181–202
Muscatine L, Porter JW, Kaplan IR (1989) Resource partitioning by reef corals as determined from stable isotope composition I. δ13C of zooxanthellae and animal tissue versus depth. Mar Biol 100:185–193
Naumann MS, Richter C, el-Zibdah M, Wild C (2009) Coral mucus as an efficient trap for picoplanktonic cyanobacteria—implications for pelagic-benthic coupling in the reef ecosystem. Mar Ecol Prog Ser 385:65–76
Naumann MS, Haas A, Struck U, Mayr C, el-Zibdah M, Wild C (2010) Organic matter release by dominant hermatypic corals of the Northern Red Sea. Coral Reefs. doi:10.1007/s00338-010-0612-7
Niggl W, Glas M, Laforsch C, Mayr C, Wild C (2009) First evidence of coral bleaching stimulating organic matter release by reef corals. In: Proc 11th int coral reef symp, Fort Lauderdale, USA, 905–911
Niggl W, Naumann MS, Struck U, Manasrah R, Wild C (2010) Organic matter release by the benthic upside-down jellyfish Cassiopea sp. fuels pelagic food webs in coral reefs. J Exp Mar Biol Ecol 384:99–106
Ogunlana M, Hooge MD, Tekle YI, Benayahu Y, Barneah O, Tyler S (2005) W. brickneri n. sp. (Acoela: Acoelomorpha) associated with corals in the Red Sea. Zootaxa 1008:1–11
Rasheed M, Badran MI, Richter C, Huettel M (2002) Effect of reef framework and bottom sediment on nutrient enrichment in a coral reef of the Gulf of Aqaba, Red Sea. Mar Ecol Prog Ser 239:277–285
Richman S, Loya Y, Slobodkin L (1975) Rate of mucus production by corals and its assimilation by the coral reef copepod Acartia negligens. Limnol Oceanogr 20:918–923
Ritchie KB (2006) Regulation of microbial populations by coral surface mucus and mucus-associated bacteria. Mar Ecol Prog Ser 322:1–14
Schlichter D, Svoboda A, Kremer BP (1983) Functional autotrophy of Heteroxenia fuscescens (Anthozoa: Alcyonaria): carbon assimilation and translocation of photosynthates from symbionts to host. Mar Biol 78:29–38
Schoeninger MJ, DeNiro MJ (1984) Nitrogen and carbon isotopic composition of bone collagen from marine and terrestrial animals. Geochim Cosmochim Acta 48:625–639
Schuhmacher H (1977) Ability of fungiid corals to overcome sedimentation. In: Proceedings of the 3rd international coral reef symposium, Miami, USA, vol 1, pp 503–509
Swart PK, Saied A, Lamb K (2005a) Temporal and spatial variation in the δ15N and δ13C of coral tissue and zooxanthellae in Montastrea faveolata collected from the Florida reef tract. Limnol Oceanogr 50:1049–1058
Swart PK, Szmant A, Porter JW, Dodge RE, Tougas JI, Southam JR (2005b) The isotopic composition of respired carbon dioxide in scleractinian corals: implications for cycling of organic carbon in corals. Geochim Cosmochim Acta 69:1495–1509
Szmant AM, Gassman NJ (1990) The effects of prolonged “bleaching” on the tissue biomass and reproduction of the reef coral Montastrea annularis. Coral Reefs 8:217–224
Trench RK, Winsor H (1987) Symbiosis with Dinoflagellates in two pelagic flatworms, Amphiscolops sp. and Haplodiscus sp. Symbiosis 3:1–22
Tyler S (2003) Platyhelminthes. The nature of a controversial phylum. http://devbio.umesci.maine.edu/styler/globalworming/platyhelm2003.html
Tyler S, Schilling S, Hooge M, Bush LF (2005) Turbellarian taxonomic database
Vacelet E, Thomassin B (1991) Microbial utilization of coral mucus in long term in situ incubation over a coral reef. Hydrobiologia 211:19–32
Wild C, Rasheed M, Werner U, Franke U, Johnstone R, Huettel M (2004a) Degradation and mineralization of coral mucus in reef environments. Mar Ecol Prog Ser 267:159–171
Wild C, Huettel M, Klueter A, Kremb SG, Rasheed M, Jørgensen BB (2004b) Coral mucus functions as an energy carrier and particle trap in the reef ecosystem. Nature 428:66–70
Wild C, Woyt H, Huettel M (2005a) Influence of coral mucus on nutrient fluxes in carbonate sediments. Mar Ecol Prog Ser 287:87–98
Wild C, Rasheed M, Jantzen C, Cook P, Struck U, Huettel M, Boetius A (2005b) Benthic metabolism and degradation of natural particulate organic matter in silicate and carbonate sands of the Northern Red Sea. Mar Ecol Prog Ser 298:69–78
Wild C, Mayr C, Wehrmann LM, Schöttner S, Naumann M, Hoffmann F, Rapp HT (2008) Organic matter release by cold water corals and its implication for fauna-microbe interaction. Mar Ecol Prog Ser 372:67–75
Wild C, Haas A, Naumann M, Mayr C, el-Zibdah M (2009a) Phase shifts in coral reefs—comparative investigation of corals and benthic algae as ecosystem engineers. In: Proceedings of the 11th international coral reef symposium, Fort Lauderdale, USA, pp 1319–1323
Wild C, Naumann MS, Haas A, Struck U, Mayer F, Rasheed M, Huettel M (2009b) Coral sand oxygen consumption and benthic-pelagic coupling in a subtropical fringing reef, Aqaba, Red Sea. Aquat Biol 6:133–142
Wild C, Niggl W, Naumann MS, Haas AF (2010a) Organic matter release by benthic coral reef organisms in the Red Sea—its effect on planktonic microbial activity and potential implication for in situ O2 availability. Mar Ecol Prog Ser. doi:10.3354/meps08653 (in press)
Wild C, Naumann MS, Niggl W, Haas AF (2010b) Carbohydrate composition of mucus released by scleractinian warm and cold water reef corals. Aquat Biol. doi:10.3354/ab00269 (in press)
Winsor L (1990) Marine Turbellaria (Acoela) from north Queensland. Mem Queensl Mus 28:785–800
Yamamuro M, Kayanne H, Minagawa M (1995) Carbon and nitrogen stable isotopes of primary producers in coral reef ecosystems. Limnol Oceanogr 40:617–621
Acknowledgments
The authors are grateful to M. Khalaf (Marine Science Station, Aqaba, Jordan); W. Niggl, A. Haas, F. Mayer and C. Jantzen (CORE, München) for technical and logistical support. C. Williamson (CORE, München) helped to improve the language of the manuscript. We thank three anonymous reviewers for their valuable comments. This study was supported by German Research Foundation (DFG) grant Wi 2677/2-1 to C.W.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by T. L. Goulet.
Rights and permissions
About this article
Cite this article
Naumann, M.S., Mayr, C., Struck, U. et al. Coral mucus stable isotope composition and labeling: experimental evidence for mucus uptake by epizoic acoelomorph worms. Mar Biol 157, 2521–2531 (2010). https://doi.org/10.1007/s00227-010-1516-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-010-1516-3