Abstract
The marine macrophyte Ulva prolifera is the dominant green-tide-forming seaweed in the southern Yellow Sea, China. Here we assessed, in the laboratory, the growth rate and nutrient uptake responses of U. prolifera to different nutrient treatments. The growth rates were enhanced in incubations with added organic and inorganic nitrogen [i.e. nitrate (NO3−), ammonium (NH4+), urea and glycine] and phosphorus [i.e. phosphate (PO43−), adenosine triphosphate (ATP) and glucose 6-phosphate (G-6-P)], relative to the control. The relative growth rates of U. prolifera were higher when enriched with dissolved organic nitrogen (urea and glycine) and phosphorus (ATP and G-6-P) than inorganic nitrogen (NO3− and NH4+) and phosphorus (PO43−). In contrast, the affinity was higher for inorganic than organic nutrients. Field data in the southern Yellow Sea showed significant inverse correlations between macroalgal biomass and dissolved organic nutrients. Our laboratory and field results indicated that organic nutrients such as urea, glycine and ATP, may contribute to the development of macroalgal blooms in the southern Yellow Sea.
Similar content being viewed by others
Introduction
Anthropogenic activities, including rapidly increasing population, excessive agricultural fertilization and intensive mariculture, have caused increasing inputs of inorganic and organic nutrients into coastal waters, which contribute to eutrophication and harmful algal blooms (HABs)1,2. Green tides, a type of HAB that can be caused by accumulation of free-floating macroalgae, have been persistent features in eutrophic coastal waters and in estuaries worldwide3,4. Large blooms of the green alga U. prolifera occurred yearly in the southern Yellow Sea, China, from 2007 to 2015. They were among the largest of such outbreaks worldwide and the large scale accumulations of macroalgae biomass caused severe environmental problems in the southern Yellow Sea5,6.
Macroalgal blooms in the southern Yellow Sea generally originate from small patches of floating green algae in coastal areas of the Jiangsu Province during mid-April to early May. These algae drift northward in the southern Yellow Sea for about 1.5 months and reach the coast near Qingdao in July or August (Supplementary Fig. S1)1,7. Previous studies on the spatiotemporal variations of dissolved inorganic and organic nutrients in the southern Yellow Sea during the period of macroalgal blooms in 2012 inferred that, although the concentrations of inorganic N and P ranged from 0.2 to 36.5 and 0 to 0.6 μM, respectively, dissolved organic nutrients may be taken up and contribute to the development and persistence of macroalgal blooms6. Indeed it was reported that, during the development of a macroalgal bloom in the southern Yellow Sea, there was an increase in the biomass of U. prolifera and area it covered and a decline in the concentrations of organic N and P6,8. It was also suggested that during the mid- to late period of the macroalgal blooms, U. prolifera almost exhausted dissolved inorganic N- and P-nutrients and continued growth may have been supported by organic nutrients9 and remineralized forms of N and P.
Macroalgal blooms in coastal waters are often the result of nutrient eutrophication, such as increased nutrient loads in river runoff and inputs from mariculture5,10. In the southern Yellow Sea, the Sheyang (33.7 °N) and Guanhe (34.3 °N) rivers discharge large quantities of inorganic and organic nutrients into the Jiangsu coastal waters, thus contributing to coastal eutrophication11. Marine aquaculture, particularly shrimp and shellfish, expanded rapidly in the Jiangsu Province during the last decade and the total area for aquaculture along the southern Yellow Sea coast has increased to 201 kha in 2011, doubling the annual production (8.42 × 105 t) relative to 2003 (4.10 × 105 t)12. In addition, ~50,000 t of fertilizers rich in organic N and P, such as fermented chicken manure, were added annually to land-based aquaculture ponds along the Jiangsu Coast5. The discharge of nutrient-rich wastewater from these aquaculture ponds could also supply organic and inorganic nutrients to nearshore coastal waters.
Previous studies have shown that U. prolifera is the most dominant green-tide-forming species in the southern Yellow Sea13. The fronds of U. prolifera generally exhibit high rates of nutrient uptake and growth in eutrophic waters and are tolerant to changes in temperature, salinity, light and desiccation14. These characteristics provide a competitive advantage to U. prolifera, relative to other macroalgae in the eutrophic coastal waters of the southern Yellow Sea15. Moreover, laboratory experiments show that U. prolifera rapidly assimilates dissolved inorganic N and P16,17, with a higher uptake rate per unit biomass of N than P16. Some field studies in the southern Yellow Sea have suggested that dissolved organic N and P may also be used by U. prolifera for growth5,6,18. Although the uptake of organic nutrients by other macroalgae has been studied and is well understood19,20, the uptake of organic N and P by U. prolifera has seldom been investigated in the laboratory21.
Planktonic algae take up NO3− and NH4+ 22 and as well as some forms of organic N23. Macroalgae take up inorganic nitrogen, urea and some free amino acids20,21. Urea is present in coastal marine waters, especially in regions impacted by anthropogenic activities, where it can account for ~5% of the dissolved N in the southern Yellow Sea during the spring of 20129. Dissolved free amino acids (DFAA), such as glycine, serine and alanine, are also common forms of organic N in seawater24. The major form of dissolved inorganic P used by algae is PO43−. Organic P can be as important as orthophosphate in supporting the growth of dinoflagellates25 and other marine phytoplankton26 when ambient PO43− concentrations are low25,26. Adenosine triphosphate (ATP) is a low-molecular-weight form of organic P in seawater and concentrations of dissolved ATP range from 0.1 to 0.6 μg L−1 27,28. Another form of low molecular weight organic-P that is rapidly cycled in algal cells is glucose 6-phosphate (G-6-P) and although it has not been measured in seawater, it is assimilated by marine phytoplankton26,29. Since ATP and G-6-P either occur in seawater or have been shown to be taken up by microalgae26,30,31,32, we used them for macroalgae in the present study.
Using laboratory experiments, we investigated the utilization of inorganic and organic N (NO3−, NH4+, urea and glycine) and P (PO43−, ATP and G-6-P) by U. prolifera and their growth rates in different nutrient treatments and to explore the idea that dissolved organic nutrients may be important in supporting the in situ growth of U. prolifera. The results of these experiments could provide indications on factors responsible for the occurrence of macroalgal blooms in the southern Yellow Sea.
Results
General results of the ANOVAs
We conducted time-course experiments with four N- and three P-substrates, where we measured changes in the biomass (measured as fresh weight) of U. prolifera, the relative growth rates of algae (Ki) and their uptake rates (V) of the various substrates. In the six two-way ANOVAs on the results of these experiments (Supplementary Tables S1–S6), there was no significant interaction (α = 0.05) between the two factors, i.e. the duration of incubations and the substrate type (P = 0.10, 0.56 and 0.29, respectively, for the N experiments; P = 0.22, 0.62 and 0.32, respectively, for the P experiments).
There was a significant effect (α = 0.05) of the N-substrate in the two-way ANOVAs conducted on biomass, Ki and V (P < 0.001, <0.001 and <0.05, respectively) and of the P-substrate in the ANOVAs conducted on biomass and V (P < 0.001 and <0.05, respectively), i.e. the effect of the P-substrate was not significant in the ANOVAs conducted on Ki (P = 0.17) (Supplementary Tables S1–S6). In the five cases where there was a significant effect of the substrate, pairwise comparisons between nutrient substrates were computed (Supplementary Tables S1–S4 and S6) and the results were detailed below. Also, there was a significant effect (α = 0.05) of the incubation duration on biomass, Ki and V in the six ANOVAs (P = 0.002, 0.014 and 0.006, respectively, for the N experiments; P < 0.001, <0.001 and <0.01, respectively, for P experiments).
For each treatment, we computed the average (Ka, % d−1) and maximum (Km, % d−1) relative growth rates of U. prolifera (Tables 1 and 2), determined as average and maximum values of Ki during the 13- and 19-day N- and P-experiments, respectively. In the four one-way ANOVAs on these values, there was a significant effect (α = 0.05) of both the N- and P-substrates (Supplementary Tables S7 and S8). In these four ANOVAs, values were significantly higher in the nutrient-enriched media than in the control group and most of the pairwise comparisons between nutrient substrates were significant (Supplementary Tables S7 and S8), as detailed below.
Effects of N on growth rates of U. prolifera
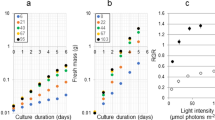
The growth responses (biomass and Ki; Fig. 1A, Supplementary Tables S1 and S2) of U. prolifera during the 13-day experiments varied with incubation duration. In the N-enriched samples, there was an increase in biomass over the incubation period and the responses of biomass production and growth rate were significantly higher when incubations were enriched with NH4+ and organic N than in the control. There were no significant differences for U. prolifera incubated with NO3− and in the control. The relative growth rates of U. prolifera incubated with urea were significantly higher than those with NO3−. From the third day onwards, macroalgal biomass increased more in the two organic-N than the two inorganic-N treatments, with the greatest biomass and Ki occurring in the urea enrichment on day 10. After about 6–10 days of incubation, the Ki of U. prolifera in the NO3− treatment remained relatively constant, whereas the Ki in the urea treatment continued to grow until about day 10 (Fig. 1A) after nutrients were depleted (Fig. 2). The Ki in the NH4+ and glycine treatments were not significantly different during the incubations (Supplementary Table S2). On day 13, U. prolifera incubated with urea and glycine had higher biomass compared with those incubated with NO3− or in the absence of added nutrients.
Most values of Ka and Km were significantly higher (α = 0.05) in the treatments with organic-N (especially urea) than inorganic-N, but there was no significant difference in the Ka of the NH4+ and glycine treatments (Table 1 and Supplementary Table S7).
Generally, the pH increased during all N-enriched incubations except in the NO3− treatment and the un-enriched control (Fig. 1B). Urea and glycice enriched incubations had the highest pH and in all treatments, the pH decreased toward the end of the experiment.
Change in concentration of the N substrates during the experiments and N uptake by U. prolifera
Ulva prolifera removed nearly all inorganic N by day 3 and the organic N by day 6 of the 13-day experiment (Fig. 2). By day 3, 97, 98, 78 and 72% of the NO3−, NH4+, urea and glycine, respectively, had been removed and the concentrations of urea and glycine remaining in the culture medium were 8.5 and 11.0 μM, respectively. By day 8, the organic-N substrates were fully depleted. Moreover, the inorganic-N concentrations in the media enriched with the two organic-N substrates, i.e. urea and glycine, remained below detection throughout the experiment.
The uptake rate and Vmax/Ks of NH4+ were the highest among the four N-substrates (Fig. 3 and Supplementary Table S9). The uptake rates of NO3− and NH4+ were significantly higher (α = 0.05) than those of urea and glycine (Supplementary Table S3).
Effects of P on growth rates of U. prolifera
The growth responses (biomass and Ki; Fig. 4A, Supplementary Tables S4 and S5) of U. prolifera during the 19-day experiments varied with incubation duration and showed overall increases in biomass over the incubation period. The initial concentrations was 2.5 μM in all P-enriched samples and there was no significant effect (α = 0.05) of the adding P on Ki. The biomass of U. prolifera in samples supplemented with ATP was significantly higher than those treated with PO43−.
The Ka and Km were significantly higher in the treatments with organic-P (especially ATP) than PO43− (Table 2 and Supplementary Table S7). The biomass of the ATP-enriched cultures was consistently the highest, except on the first day of the experiment when biomass was higher in the PO43− treatment (Fig. 4A). From day 2 onwards, the biomass in the two organic P-treatments (ATP and G-6-P) exceeded that in the inorganic P-treatment, particularly after day 13 (Fig. 4A).
The pH in all incubations increased from about day 1 to day 13, after which they decreased in both the control and all treatments (Fig. 4B).
Change in concentration of the P substrates during the experiments and P uptake by U. prolifera
Ulva prolifera removed all the PO43− by day 2 and the organic-P by day 10 of the 19-day incubation (Fig. 5). By day 1, 92, 54 and 52% of the PO43−, ATP and G-6-P, respectively, had been removed. The two organic substrates decreased more slowly than PO43−, but were totally depleted on day 10. Moreover, the dissolved inorganic phosphorus concentrations in the media enriched with the two organic-P substrates, i.e. ATP and G-6-P, remained below detection throughout the experiment.
The uptake rate of PO43− and the Vmax/Kswere the highest among the three P-substrates (Fig. 6 and Supplementary Table S10). The uptake rate of PO43− was significantly higher (α = 0.05) than those of ATP and G-6-P (Supplementary Table S6).
Effect of the changes in pH on the uptake of dissolved inorganic carbon (DIC) by U. prolifera in N and P experiments
The pH in the southern Yellow Sea ranged from 7.73 to 8.15, 7.99 to 8.35 and 7.98 to 8.65 in the early (11–26 April), mid (30 April–31 May) and late (13–23 June) periods of green tides in 2010, with average values of 7.85 ± 0.06, 8.07 ± 0.08, 8.14 ± 0.06, respectively33. In the Supplementary Material, we used the pH to calculate potential DIC uptake by U. prolifera at the beginning of incubations. Based on pH in the N- and P-experiments and in the field, we calculated the effect of differences in pH on the uptake of DIC by U. prolifera at the beginning of incubations relative to field conditions in the southern Yellow Sea. Results showed that the DIC uptake by U. prolifera was potentially ~10% lower in the incubation containers at the beginning of experiments than in the southern Yellow Sea. Similarly, the DIC uptake by U. prolifera at the beginning of the N- and P-experiments varied by ~3 and ~1%, respectively, between the highest and lowest initial pH values.
Relationship between concentrations of organic nutrients and macroalgal blooms in the southern Yellow Sea
To examine the relationship between dissolved organic-N nutrients and macroalgal blooms in the southern Yellow Sea, we plotted the biomass of U. prolifera in surface water as a function of the field concentrations of urea and dissolved organic N and P, during four periods between April and June 2012 (Supplementary Fig. S2A–L). There were significant inverse correlations (Spearmann rho, α = 0.05) between macroalgal biomass with all nutrients during the 31 May–9 June 2012 period (Supplementary Fig. S2D,H,L). There were also significant inverse correlations with the urea concentrations during the 11–21 May and 25–29 May 2012 periods (Supplementary Fig. S2B,C).
Discussion
Methodological considerations
We examine here three methodological aspects of our results. These are the precautionary addition of nutrients in the experiments, the pH in the nutrient-amended treatments and the possible effect of bacterial uptake on nutrient concentrations during the incubations.
There were two important differences between the results of experiments with N- and P-enrichments. Firstly, the nutrient type had a significant effect on Ki in the N-experiments but not in the P-experiments (Supplementary Tables S2 and S5). Secondly, the biomass in the controls did not increase during the N-experiments but did during the P-experiments (Figs 1A and 4A). In all experiments, we added 10 μM PO43− to the N-experiments and 100 μM NO3− to the P-experiments, including the controls. This precautionary addition of PO43− to the N-experiments was done to prevent P from limiting growth during the N-amended treatments and vise-versa. The different responses of the controls in the two sets of experiments suggested that the NO3− concentration added in the P-experiments stimulated Ki of U. prolifera. However, this possible methodological problem did not prevent the added P-substrate from having a significant effect on the biomass and V in experiments with P-treatments (Supplementary Tables S4 and S6).
Temporal variations in the pH of the growth medium reflected the growth of U. prolifera (Figs 1B and 4B). Indeed, increased pH likely corresponded to a decrease in dissolved CO2 caused by enhanced photosynthesis and biomass production due to amendment with limiting nutrients34. Given that there was no difference in the pH of the culture medium before addition of the N- and P- amendments, these were likely the cause of the initial pH differences among treatments (Figs 1B and 4B). The initial pH in the two sets of experiments (i.e. 8.61–8.87 and 8.86–8.96 in the N- and P-incubations, respectively) was higher that of coastal seawater in the southern Yellow Sea (approximately 8.0)33, but previous studies indicated that U. prolifera could grow well at pH between 6 and 10 and their optimal pH for the growth was 8 to 935,36. The higher pH in the incubation bottles than in coastal seawater at the beginning of our experiments could have lead to a 10% lower DIC uptake by U. prolifera in the incubations than in the natural environment. It was not possible use the same approach to derive changes in DIC uptake from changes in pH during the course of the experiments because constant alkalinity cannot be assumed in our incubation containers37. The observed decrease in pH after maximum values in mid-experiments suggested that the cultured were probably not, or at least not severely carbon limited at the end of the experiments. The relative difference between the highest and lowest DIC uptake at the beginning of N and P experiments, derived from differences in pH, was ~3% and ~1%, respectively. Such small differences did not likely influence much carbon uptake in different experiments.
The fact that U. prolifera grew with both inorganic or organic N- or P-nutrients (Tables 1 and 2) and their decrease during the experiments (Figs 2 and 5) could be interpreted in at least three different ways: (1) the nutrients were taken up directly by U. prolifera, (2) the organic nutrients were assimilated by bacteria and transformed by bacteria into inorganic N- or P-forms that were subsequently taken up by the macroalgae, or (3) the inorganic or organic N and P were taken up by bacteria (attached to the macroalgae or free-living) and not by U. prolifera. We interpreted the observed decreases in nutrient concentrations during the experiments (Figs 2 and 5) as uptake by U. prolifera (Figs 3 and 6) and not by attached free-living bacteria or microalgae. Bacteria and the microalgae attached to U. prolifera and in the culture media were killed or their numbers were largely reduced before incubation by cleaning and antibiotic treatments. Although some bacteria might have grown during the incubations, their influence on nutrient uptake was small relative to that of the macroalgae (Supplementary Fig. S3). The pretreatment of U. prolifera with antiobitics38, the subsequent washing in sterile seawater and the use of sterile glassware and techniques throughout the experiments should have avoided introducing heterotrophic bacteria into the cultures and minimized the growth of epiphytic bacteria that may have survived the antibiotic treatment. Nevertheless, we assessed the potential uptake of nutrients by bacteria during the incubations by simulating bacterial-mediated nutrient uptake at three representative growth rates (Supplementary Material). In the modeled situation, the bacterial-mediated decline in nutrients was greater at the higher bacterial growth rates and most of the uptake of N and P by bacteria occurred after day 6 to 12 (Supplementary Fig. S3). This was different from our observations during the incubations with U. prolifera, where >90% of the N and P uptake occurred by days 2 to 4 and 1 to 5, respectively (Figs 2 and 5). We concluded that the effect of bacterial uptake on the decline of N and P during the incubations with U. prolifera was small and did not biased the interpretation of the experimental results.
Growth of U. prolifera in experiments
The biomass and the relative growth rate of U. prolifera increased for all inorganic and organic N- and P-additions (Figs 1 and 4), indicating that U. prolifera used various N- or P-nutrient substrates for growth. The highest growth responses were observed in two of the organic N- and P-treatments, i.e. urea and ATP (Km, Tables 1 and 2), during the mid-to-late incubation periods (Figs 1 and 4). The combination of these laboratory results on the uptake and growth of U. prolifera using organic N and P with the field data of 31 May to 9 June 2012 showing significant inverse correlations between U. prolifera biomass and the concentrations of organic nutrients (Supplementary Fig. S2) led us to suggest that organic nutrients may have been used by U. prolifera for growth at sea, especially when inorganic concentrations at sea were low during the mid- to late period of the macroalgal blooms6,18.
Uptake of inorganic and organic nutrients by U. prolifera
Nutrient uptake rates by macroalgae have often been described as a saturating function of substrate concentration using the Michaelis–Menten equation39. We used the Michaelis–Menten relationship to describe the uptake of inorganic and organic N-nutrient by U. prolifera (Fig. 3). The Vmax/Ks for the uptake of urea and glycine were lower than those for NO3− and NH4+ (Supplementary Table S9), which suggested more efficient uptake of inorganic than organic N-nutrients by the U. prolifera fronds. We also found that the growth rate of U. prolifera was significantly higher in the urea and glycine and NH4+ compared to NO3− treatment (Supplementary Table S2). This was likely due to the differences in N uptake kinetics of U. prolifera. U. prolifera had a higher affinity for NH4+ than NO3− in seawater16,39, which was owing to the lower amount of energy required for assimilation of NH4+ than NO3− given that NO3− needs to be reduced to NH4+ by nitrate reductase before being assimilated40. Moreover, previous studies have shown that some organic-N compounds, especially organic-N released at the decline of the spring phytoplankton bloom, were likely more easily assimilated by macroalgae than NO3− 41,42,43.
Previous laboratory experiments have shown that U. prolifera take up N at a markedly higher Vmax/Ks than P16 and our present results show that U. prolifera can take up both N- and P-nutrients, including some forms of organic-P (ATP and G-6-P, Fig. 6; Supplementary Table S10). In the southern Yellow Sea from April to June 2012, the concentrations of PO4-P and organic-P ranged from undetectable to 0.6 μM and from undetectable to 1.2 μM, respectively and the organic-P concentrations can account for ~65% of the total dissolved phosphorus6. Since the Vmax/Ks value in experiments with PO43− was higher than the values for ATP and G-6-P (Fig. 6, Supplementary Table S10), we concluded that U. prolifera preferentially assimilated inorganic- over organic-P substrates.
Our laboratory results showed a significant (α = 0.05) increase in U. prolifera biomass when enriched with ATP and G-6-P, but not in treatments enriched with PO43− (Supplementary Table S4, control vs. treatments). Previous investigations had shown that P-limited phytoplankton could take up labile and low molecular weight organic-P from seawater especially when PO43− concentrations were low25,44,45. ATP, one of labile organic-P forms, is the powerhouse for nutrient uptake by phytoplankton as it can store and provide energy for nutrient assimilation46,47. We hypothesized that in our experiment the significant increase in fresh weight of U. prolifera under treatments enriched with organic-P may result in part from the enhanced N uptake driven by organic-P addition in the medium45.
Among the most important findings of our laboratory experiments was that the bloom-forming macroalga U. prolifera took up both inorganic and organic nutrients, supporting the idea that organic nutrients may contribute to the development of green macroalgal blooms in the southern Yellow Sea.
Conclusion
During the nine successive years of massive blooms of green macroalgae in the southern Yellow Sea, these blooms always started in coastal waters of the Jiangsu Province during mid-April. The present study provides experimental evidence that is consistent the idea that organic nutrients may be involved in the occurrence of green-tide blooms in the southern Yellow Sea. The role of organic nutrients could be particularly important when inorganic nutrients are low, i.e. during the mid to late period of the annual development of the green-tide blooms18. This suggests that controlling the discharge of organic nutrients, such as unused feed and organic excreta from rivers and mariculture ponds, may reduce the annual occurrence of harmful macroalgal blooms in the southern Yellow Sea.
Materials and Methods
Algal material collection and pre-treatment
Fronds of U. prolifera were sampled from Porphyra yezoensis mariculture rafts in Rudong (121°5′20.4″N; 32°40′51.6″E), Jiangsu Province, China, in April 2013. The intact and healthy algal fronds collected in situ were stored at approximately 4 °C for transport to the laboratory (within 48 h). Upon arrival, the fronds were gently washed several times with sterile seawater (autoclave, 120 °C, 20 min), cleaned with 1% sodium hypochlorite for 1–2 min and rinsed with sterile artificial seawater. Epiphytes and visible particles attached to the fronds were removed, after which the fronds were placed overnight in an incubator (at 15 °C, in darkness) in sterile artificial seawater with 1 ml L−1 of GeO2 dissolved in milli-Q water (0.5 mg mL−1) to inhibit the growth of diatoms and antibiotics (4% Kanamycin) to kill bacteria. The seaweeds were then incubated (in a GXZ-380B illumination incubators, Ningbo, Zhejiang, China, at 15 °C, under an irradiance of 80 μmol photons m−2 s−1 with a 12:12 h light:dark cycle) in sterile artificial seawater (ASW) enriched with f/2 medium during 2 days48. Four days before the experiments, the U. prolifera fronds were incubated in aged ASW (salinity of 35.0, pH of 8.1) supplemented with modified f/2 medium (no N, P or Si and supplemented with trace elements and vitamins) to decrease the N or P content in U. prolifera tissues. The incubation containers were washed with 20% sulfuric acid (H2SO4), rinsed several times with Milli-Q water (pH = 7.0), sterilized (autoclave, 120 °C, 20 min) and dried in a muffle furnace at 450 °C during 4 h to prevent nutrient contamination. They were closed with Parafilm™ and incubated as explained above.
Experimental design
We investigated the effects of N and P substrates on the growth and uptake rate of U. prolifera, during laboratory incubations. Ulva prolifera were incubated in 3-L incubation containers containing 1800 mL media (aged ASW + modified f/2 medium; see above). The initial algal biomass (fresh weight) in each incubation container was 0.3 g L−1. All experiments were conducted under the same clean but not sterile conditions, described above. The incubation containers were sealed with Parafilm™, which was briefly opened at the times of sampling. Three independent, parallel incubations (triplicates) were performed for each control and nutrient treatment. The pH of incubated samples was measured with a handheld pH meter (PB-10, Sartotius, USA; precision ± 0.01 pH unit). The pH of the medium was the same in all incubation containers before adding nutrients. The initial (day 0) pH of each treatment was measured after adding the N or P substrates and the U. prolifera fronds to the medium. Water samples for nutrient analyses were filtered on GF/F filters, which had been pretreated by heating at 450 °C for 4 hours and 5 ml were stored in polyethylene flasks at −20 °C. Before taking the water samples, the algal frond inside each incubation container was collected and drained to remove excess water and its biomass (determined as fresh weight) was determined immediately. On the last day of incubations, after measuring their wet-weight biomass, the dry weight of macroalgal tissues was determined by drying the incubated fronds at 55 °C for 72 h.
The growth of U. prolifera was measured as a function of added dissolved inorganic and organic N and P. The four N substrates were NO3− (NaNO3), NH4+ (NH4Cl), urea (CO(NH2)2) and glycine (C2H5NO2). In each treatment, a single N substrate was added to N-depleted sterile ASW and then N concentration was adjusted to 40 μM. The control group consisted of incubations without N addition. The treatments and control received 10 μM PO43− (KH2PO4) to prevent P limitation of macroalgal growth during the experiment39,49. To determine N concentrations, 5 mL of incubation medium were collected on days 0, 0.5, 1, 2, 3, 4, 6, 8, 10 and 13. The P-dependent growth of on U. prolifera was measured for the three PO43− (NaH2PO4), ATP (C10H14N5Na2O13P3) and G-6-P (C6H12NaO9P). In each treatment, a single P substrate was added to P-depleted sterile ASW and the P concentration was adjusted to 2.5 μM. The control group consisted of incubations without P addition. The control and treatments receive 100 μM of NO3− to prevent N limitation of macroalgal growth during the experiment39,49. To determine P concentrations, 5 mL of incubation medium were collected on days 0, 0.25, 0.5, 1, 2, 4, 7, 10, 13, 16 and 19.
Sample analysis
Spectrophotometric analysis of NO3-N, NH4-N and PO4-P was performed using an AutoAnalyzer (BRAN and LUEBBE AA3, Germany) after the water samples had been thawed to room temperature. Total dissolved phosphorus (TDP) was measured by persulfate oxidation50 and dissolved organic phosphorus was obtained by subtracting dissolved inorganic phosphorus from TDP. The analytical precision of the NO3-N, NH4-N, PO4-P and TDP determinations were 0.04, 0.03, 0.02 and 0.02 μM, respectively. Urea concentrations in the samples were determined manually based on the reaction of urea with diacetylmonoxime and the analytical precision was 0.03 μg at urea-N 1−1 51. Free amino acids were measured by pre-column derivatization high-performance liquid chromatography and the analytical precision was 4 to 29 fmol for individual amino acids52.
Calculations
To analyze the growth responses of U. prolifera to various inorganic and organic nutrient treatments, we calculated the relative growth rates of macroalgal fronds with the following equation53:

where Kiis relative growth rate of U. prolifera, ln is the natural logarithm, Fw is the final fresh weight (i.e. at the end of the sampling interval), Iw is the initial fresh weight (i.e. at the beginning of the sampling interval) and T is the duration of the incubation interval (e.g. T = 3 days when the incubated water was sampled every third day).
The uptake rates for inorganic and organic N or P substrate were calculated using the observed decline in nutrient concentrations during each sampling interval with the following equation:

where V is the uptake rate of the nutrient substrate (μmol g(dw)−1 h−1), C0 and Ct are the nutrient concentrations (μM) at the beginning and at the end of the sampling interval, respectively, Vt is the water volume (L) at the end of the sampling interval, T is the duration (h) of the sampling interval and N is the dry weight (g) of macroalgae. Since the life cycle of macroalgae, such as U.prolifera, is longer than that of phytoplankton54,55, we used in this study longer incubation times than are typical of microalgal nutrient uptake experiments. Nitrogen and phosphorus uptake by U. prolifera was estimated from the decline in ambient nitrogen between incubations days 0, 0.5, 1, 2, 3, 4, 6 and 8 (i.e. 0, 12, 24, 48, 72, 96, 144 and 192 h) and 0, 0.25, 0.5, 1, 2, 4 and 7 (i.e. 0, 6, 12, 24, 48, 96, 168 h), respectively.
The Michaelis–Menten equation, originally developed to describe enzyme kinetics, has been used to describe the nutrient uptake and growth response of macroalgae39:

where V is the uptake rate of the nutrient substrate computed for each sampling interval (eq. 2) between 0 and 192 h and 0 and 168 h for the N- and P-experiments, respectively and C is the nutrient concentration (μM) measured at the end of the sampling interval. In equation (3), Vmax and Ks are the maximum uptake rate and the substrate concentration at which uptake proceeds at half the maximum rate (half-saturation constant), respectively. The kinetic parameters Vmax and Ks were obtained for each N and P substrate by fitting equation (3) to hyperbolic tangent plots of uptake rates versus nutrient concentrations using SigmaPlot 12.5. The ratio Vmax/Kshas been used an index of affinity, were high values imply high substrate affinity56.
Field data from the southern Yellow Sea
We used published and unpublished field data on urea, organic-N, organic-P and U. prolifera biomass to determine the relationship between macroalgal blooms and nutrients at sea. These data were from four cruises conducted in coastal waters of the southern Yellow Sea (32–36° N, 120–124° E) between 27 April and 9 June 2012. The urea data are reported for the first time in the present study, whereas the other data had been reported in two previous studies, i.e. organic-N and organic-P in one study6 and U. prolifera biomass in another study8. The relationships between biomass and the three nutrients are analyzed here for the first time.
Statistical analysis
ANOVA was used to assess the effect of different N and P treatments on the growth responses and nutrient uptake rates of U. prolifera. Firstly, biomass, Ki and V were analyzed using two-way ANOVA, where the two factors were incubation duration and the nutrient treatment. In cases where there were both no significant interaction between the two factors and a significant effect of the nutrient treatment, a posteriori comparisons between pairs of nutrient treatments were conducted to identify which of the treatments differed significantly from others (Holm-Sidak tests). Secondly, Ka and Km were analyzed using one-way ANOVA, where the factor was the nutrient treatment. In cases where there was a significant effect of the nutrient treatment, a posteriori comparisons between pairs of nutrient treatments were conducted as done in two-way ANOVA.
Assumptions of homogeneity of variance and normality in the ANOVAs were assessed by scatter plots of residuals and normal curves of residuals, respectively (Holm-Sidak test, SigmaPlot 12.5). Spearman rho correlation was used to assess if there was a significant monotonic relationship between pairs of variables. We used Spearman rho correlation because it does not assume linear relationships between pairs of variables. Statistical analyses were performed using SigmaPlot 12.5. The significance level was α = 0.05 for all tests unless otherwise stated.
Additional Information
How to cite this article: Li, H. et al. Growth responses of Ulva prolifera to inorganic and organic nutrients: Implications for macroalgal blooms in the southern Yellow Sea, China. Sci. Rep. 6, 26498; doi: 10.1038/srep26498 (2016).
References
Liu, D. et al. Recurrence of Yellow Sea green tide in June 2009 confirms coastal seaweed aquaculture provides nursery for generation of macroalgae blooms. Mar. Pollut. Bull. 60, 1423–1432 (2010).
Howarth, R. et al. Coupled biogeochemical cycles: eutrophication and hypoxia in temperate estuaries and coastal marine ecosystems. Front Ecol. Environ. 9, 18–26 (2011).
Choi, H. G. et al. Effects of temperature and salinity on the growth of Gracilaria verrucosa and G. chorda, with the potential for mariculture in Korea. J. Appl. Phycol. 18, 269–277 (2006).
Guidone, M. & Thornber, C. S. Examination of Ulva bloom species richness and relative abundance reveals two cryptically co-occurring bloom species in Narragansett Bay, Rhode Island. Harmful Algae 24, 1–9 (2013).
Liu, D. et al. The world’s largest macroalgae bloom in the Yellow Sea, China: formation and implications. Estuar. Coast. Shelf S. 129, 2–10 (2013).
Shi, X. Y., Qi, M. Y., Tang, H. J. & Han, X. R. Spatial and temporal nutrient variations in the Yellow Sea and their effects on Ulva prolifera blooms. Estuar. Coast. Shelf S. 163, 36–43 (2015).
Keesing, J. K., Liu, D., Fearns, P. & Garcia, R. Inter- and intra-annual patterns of Ulva prolifera green tides in the Yellow Sea during 2007–2009, their origin and relationship to the expansion of coastal seaweed aquaculture in China. Mar. Pollut. Bull. 62, 1169–1182 (2011).
Liu, X. Q., Li, Y., Wang, Z. L., Zhang, Q. C. & Cai, X. Q. Cruise observation of Ulva prolifera bloom in the southern Yellow Sea, China. Estuar. Coast. Shelf S. 163, 17–22 (2015).
Gao, S., Shi, X. Y. & Wang, T. Variation of nutrient concentrations at the inshore coastal area of northern Jiangsu province and the occurrence of green tide caused by Ulva prolifera. Enviro. Sci. 33, 2204–2209 (2013). (in Chinese with English abstract).
Pérez-Mayorga, D. M. et al. Nitrogen uptake and growth by the opportunistic macroalga Ulva lactuca (Linnaeus) during the internal tide. J. Exp. Mar. Biol. Ecol. 406, 108–115 (2011).
Li, H. M., Zhang, C. S., Han, X. R. & Shi, X. Y. Changes in concentrations of oxygen, dissolved nitrogen, phosphate and silicate in the southern Yellow Sea, 1980–2012: Substrates and seaward gradients. Estuar. Coast. Shelf. S. 163, 44–55 (2015).
Pang, S. J. et al. Tracking the algae origin of the Ulva bloom in the Yellow Sea by a combination of molecular, morphological and physiological analyses. Mar. Environ. Res. 69, 207–215 (2010).
Liu, F., Pang, S. J., Zhao, X. B. & Hu, C. M. Quantitative, molecular and growth analyses of Ulva microscopic propagules in the coastal sediment of Jiangsu province where green tides initially occurred. Mar. Environ. Res. 74, 56–63 (2012).
Taylor, R., Fletcher, R. L. & Raven, J. A. Preliminary studies on the growth of selected green tide algae in laboratory culture: effects of irradiance, temperature, salinity and nutrients on growth rate. Bot. Mar. 44, 327–336 (2011).
Liu, F. et al. Ulva diversity in the Yellow Sea during large-scale green algal blooms in 2008–2009. Phycol. Res. 58, 270–279 (2010).
Fan, X. et al. The effect of nutrient concentrations, nutrient ratios and temperature on photosynthesis and nutrient uptake by Ulva prolifera: implications for the explosion in green tides. J. Appl. Phycol. 26, 537–544 (2014).
Runcie, J. W., Ritchie, R. J. & Larkum, A. W. D. Uptake kinetics and assimilation of phosphorus by Catenella nipae and Ulva lactuca can be used to indicate ambient phosphate availability. J. Appl. Phycol. 16, 181–194 (2004).
Zhou, M. J., Liu, D. Y., Anderson, D. M. & Valiela, I. Introduction to the Special Issue on green tides in the Yellow Sea. Estuar. Coast. Shelf. S. 163, 3–8 (2015).
Fujita, R. M. The role of nitrogen status in regulating transient ammonium uptake and nitrogen storage by macroalgae. J. Exp. Mar. Biol. Ecol. 92, 283–301 (1985).
Tarutani, K., Niimura, Y. & Uchidaa, T. Short-term uptake of dissolved organic nitrogen by an axenic strain of Ulva pertusa (Chlorophyceae) using 15N isotope measurements. Bot. Mar. 47, 248–250 (2004).
Li, J. P. & Zhao, W. H. Effects of nitrogen specification and culture method on growth of Enteromorpha prolifera. Chin. J. of Oceanol. Limn. 29, 874–882 (2011).
Goldman, J. C. & Glibert, P. M. Nitrogen in the Marine Environment. (eds Carpenter, E. J., Capone, D. G. ) Ch. 7, 233–273 (New York, 1983).
Akram, A. K. M., Joseph, K. J. & Menon, N. R. Contribution of size fractions of planktonic algae to primary organic productivity in the coastal waters of Cochin-south west coast of India. China Law 50, 550–554 (1998).
Tada, K., Tada, M. & Maita, Y. Dissolved free amino acids in coastal seawater using a modified fluorometric method. J. Oceanogr. 54, 313–321 (1998).
Rivkin, R. B. & Swift, E. Phosphorus metabolism of oceanic dinoflagellates: phosphate uptake, chemical composition and growth of Pyrocystis noctiluca. Mar. Biol. 88, 189–198 (1985).
Cembella, A. D., Antia, N. J. & Harrison, P. J. The utilization of inorganic and organic phosphorous compounds as nutrients by eukaryotic microalgae: A multidisciplinary perspective: Part I. Crit. Rev. Microbiol. 10, 317–391 (1984).
Hodson, R. E. & Azam-Proc, F. Determination and biological significance of dissolved ATP in seawater. Methodol. Symp. 2, 1–5 (1977).
Azam, F., Hodson, R. E. & Dissolved A. T. P. in the sea and its utilisation by marine bacteria. Nature 267, 696–698 (1977).
Kuenzler, E. J. Glucose-6-phosphate utilization by marine algae. J. Phycol. 1, 156–164 (1965).
Huang, B. Q., Ou, L. J., Hong, H. S., Luo, H. W. & Wang, D. Z. Bioavailability of dissolved organic phosphorus compounds to typical harmful dinoflagellate Prorocentrum donghaiense Lu. Mar. Pollut. Bull. 51, 838–844 (2005).
Ou, L. J., Huang, X. Y., Huang, B. Q., Qi, Y. Z. & Lu, S. H. Growth and competition for different forms of organic phosphorus by the dinoflagellate Prorocentrum donghaiense with the dinoflagellate Alexandrium catenella and the diatom Skeletonema costatum s.l. Hydrobiologia 754, 29–41 (2015).
Pang, Y., Li, B. & Lü, S. H. Effects of different kinds of phosphorus substrates on growth and Alkaline Phosphatase Activity (APA) of Chattnella marina (Raphidophyceae). J. of Anhui Agri. Sci. 38, 9146–9148 (2010). (in Chinese with English abstract).
Gao, S., Fan, S. L., Han, X. R., Li, Y. & Shi, X. Y. Relations of Ulva prolifera blooms with temperature, salinity, dissolved oxygen and pH in the southern Yellow Sea. China Environ. Sci. 34, 213–218 (2014). (in Chinese with English abstract).
Schmidt, L. E. & Hansen, P. J. Allelopathy in the prymnesiophyte Chry-sochromulina polyepis: effect of cell concentration, growth phase and pH. Mar. Ecol-progr. Ser. 216, 67–81 (2001).
Wang, J. W., Yan, B. L., Lin, A. P., Hu, J. P. & Shen, S. D. Ecological factor research on the growth and induction of spores release in Ulva prolifera (Chlorophyta). Mar. Sci. Bull. 26, 60–65 (2007).
Wu, H. X., Xu, A. G. & Wu, N. M. Preliminary study on experimental ecology of Ulva prolifera. J. Zhejiang Ocean Univ. 19, 230–234 (2000). (in Chinese with English abstract).
Axelsson, L. Changes in pH as a measure of photosynthesis by marine macroalgae. Mar. Biol. 97, 287–294 (1988).
Yang, J. L., Satuito, C. G., Bao, W. Y. & Kitamura, H. Larval settlement and metamorphosis of the mussel Mytilus galloprovincialis on different macroalgae. Mar. Biol. 152, 1121–1132 (2007).
Luo, M. B., Liu, F. & Xu, Z. L. Growth and nutrient uptake capacity of two co-occurring species, Ulva prolifera and Ulva linza. Aquat. Bot. 100, 18–24 (2012).
Eppley, R. W., Rogers, J. N. & McCarthy, J. J. Half-saturation constants for uptake of nitrate and ammonium by marine phytoplankton. Limnol. Oceanogr. 14, 912–920 (1969).
Jones, A. B., Dennison, W. C. & Stmart, G. R. Macroalgal responses to nitrogen source and availability: amino acid metabolic profiling as a bioindicator using Gracilaria edulis (Rhodophyta). J. Phycol. 32, 757–766 (1996).
Neubauer, A. T. A. Benthic decomposition of Zostera marinaroots: a controlled laboratory experiment. J. Exp. Mar. Biol. Ecol. 313, 105–124 (2004).
Merceron, M., Antoine, V., Auby, I. & Morand, P. In situ growth potential of the subtidal part of green tide forming Ulva spp. stocks. Sci. Total Environ. 384, 293–305 (2007).
Taft, J. L., Taylor, W. R. & McCarthy, J. Uptake and release of phosphorus by phytoplankton in the Chesapeake Bay estuary, USA. Mar. Biol. 33, 21–32 (1975).
Cotner, J. B. & Wetzel, R. G. Uptake of dissolved inorganic and organic phosphorus compounds by phytoplankton and bacterioplankton. Limnol. Oceanogr. 37, 232–243 (1992).
Palmgren, M.G. Plant Plasma Membrane H+-ATPases: powerhouses for nutrient uptake. Plant Physiol. Plant Mol. Biol. 52, 817–845 (2001).
Schmitz, K. & Srivastava, L. M. The enzymatic incorporation of 32P into ATP and other organic compounds by sieve-tube sap of Macrocystis integrifolia bory. Planta (Berl.) 116, 85–89 (1974).
Berges, J. A., Franklin, D. J. & Harrison, P. J. Evolution of an artificial seawater medium: improvements in enriched seawater, artificial water over the last two decades. J. Phycol. 37, 1138–1145 (2001).
Björnsäter, B. R. & Wheeler, P. A. Effect of nitrogen and phosphorus supply on growth and tissue composition of Ulva fenestra and Enteromorpha intestinalis (Ulvales, Chlorophyta). J. Phycol. 26, 603–611 (1990).
Valderrama, J. C. The simultaneous analysis of total nitrogen and total phosphorus in natural waters. Mar. Chem. 10, 109–122 (1981).
Mulvenna, P. F. & Savidge, G. A modified manual method for the determination of urea in seawater using diacetylmonoxime reagent. Estuar. Coast. Shelf. S. 34, 429–438 (1992).
Kaiser, K. & Benner, R. Hydrolysis-induced racemization of amino acids. Limnol. Oceanogr. 3, 318–325 (2005).
Abreu, M. H., Pereira, R., Buschmann, A. H., Sousa-Pinto, I. & Yarish, C. Nitrogen uptake responses of Gracilaria vermiculophylla (Ohmi) Papenfuss under combined and single addition of nitrate and ammonium. J. Exp. Mar. Biol. Ecol. 407, 190–199 (2011).
Li, Y. et al. Tempo-spatial distribution and species diversity of green algae micro-propagules in the Yellow Sea during the large-scale green tide development. Harmful Algae 39, 40–47 (2014).
Song, W. et al. Temporal and spatial distributions of green algae micro-propagules inthe coastal waters of the Subei Shoal, China. Estuar. Coast. Shelf S. 163, 29–35 (2015).
Healey, F. P. Slope of the Monod equation as an indicator of advantage in nutrient competition. Microb. Ecol. 5, 281–286 (1980).
Acknowledgements
The authors thank Prof. Zongling Wang and his group for providing the U. prolifera biomass data in the southern Yellow Sea from 27 April to 9 June 2012. The authors also thank colleagues from the Laboratory of Marine Pollution Eco-chemistry at the Ocean University of China for their help during field work. This study was jointly supported by a Key R&D Project of the Shandong Province (2015GSF115036) and the National Programme on Global Change and Air-Sea Interaction (GASI–03–01–02–05), the National Basic Research Program of China (No.2013CB955700). The 2015 President’s International Fellowship Initiative of the Chinese Academy of Sciences (CAS PIFI 2015VEA011) supported the participation of LL and CAS PIFI 2016VTA038 and the Natural Science and Engineering Research Council of Canada supported the participation of RBR.
Author information
Authors and Affiliations
Contributions
H.L. performed the experiments. Y.Z. contributed to the data analysis and interpretation. X.H. prepared some figures. X.S. designed the project. L.L. contributed to the ANOVA analyses and reviewed the manuscript. R.B.R. contributed to development of the simulation model of bacterial nutrient uptake and revised the manuscript. All authors wrote the paper.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Li, H., Zhang, Y., Han, X. et al. Growth responses of Ulva prolifera to inorganic and organic nutrients: Implications for macroalgal blooms in the southern Yellow Sea, China. Sci Rep 6, 26498 (2016). https://doi.org/10.1038/srep26498
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep26498
This article is cited by
-
Study of screening, transport pathway, and vasodilation mechanisms on angiotensin-I converting enzyme inhibitory peptide from Ulva prolifera proteins
Acta Oceanologica Sinica (2023)
-
Characterization of SARS-COV-2 main protease inhibitory peptides from Ulva prolifera proteins
Journal of Oceanology and Limnology (2023)
-
The nitrogen bioextraction potential of nearshore Saccharina latissima cultivation and harvest in the Western Gulf of Maine
Journal of Applied Phycology (2021)
-
Dynamic Diurnal Changes in Green Algae Biomass in the Southern Yellow Sea Based on GOCI Images
Journal of Ocean University of China (2020)
-
Deep Water Nutrient Supply for an Offshore Ulva sp. Cultivation Project in the Eastern Mediterranean Sea: Experimental Simulation and Modeling
BioEnergy Research (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.