-
PDF
- Split View
-
Views
-
Cite
Cite
Lien T. Luong, Harry K. Kaya, Sexually transmitted nematodes affect spermatophylax production in the cricket, Gryllodes sigillatus, Behavioral Ecology, Volume 16, Issue 1, Jan./Feb. 2005, Pages 153–158, https://doi.org/10.1093/beheco/arh146
Close - Share Icon Share
Abstract
Parasites can influence various aspects of host reproduction and mating, including spermatophore production. In the cricket, Gryllodes sigillatus, males transfer to females a two-part spermatophore containing a sperm-filled ampulla and a gelatinous spermatophylax (nuptial gift). Here we investigate the effects of a sexually transmitted nematode on male spermatophylax production. Sexually transmitted diseases (STDs) have the potential to reduce host fertility or fecundity in insect hosts. To our knowledge this is the first empirical study on the effects of an insect STD on the reproductive physiology of a male host. Our results indicate that infected males produced significantly smaller spermatophylaces than healthy males; this effect was more apparent for smaller males. Spermatophylax size was inversely correlated with the intensity of infection. Spermatophylax replacement time, the time between producing the first and second spermatophylax, did not differ significantly between infected and healthy males. This parasite-mediated reduction in spermatophylax size may be a direct consequence of the physiological stress of parasitism or parasite manipulation.
Sexually transmitted diseases (STDs) are widespread in nature, occurring in many plant and animal taxa (Lockhart et al., 1996; Smith and Dobson, 1992). STDs have often been thought of as a subset of other infectious diseases, despite the fact that they have potentially different ecological and evolutionary outcomes than other infectious diseases (Knell, 1999; Lockhart et al., 1996). Sexually transmitted parasites may influence the evolution of host mating behavior, reproductive physiology, and mate choice (Freeland, 1976; Hamilton, 1990; Sheldon, 1993; Thrall et al., 1997).
Induced sterility (e.g., parasitic castration and fecundity reduction) by non-STDs has been reported in several insect taxa (Baudoin, 1974; Hurd, 2001a,b). Adverse effects on host reproduction include damage to gonads, hormonal disruption, or morphological anomalies resulting in the inability to mate and reproduce (Brown and Reed, 1997; Hurd, 2001b). In addition, parasites may influence the production of viable sperm or the development of male accessory glands (Beckage, 2002; Carver and Hurd, 1998).
There are, however, relatively few studies on the effects of an STD on insect reproduction. The most commonly reported STD-induced pathology among insects is reduced host fertility or fecundity. Hurst et al. (1995) showed that the beetle, Adalia bipunctata, infected with a sexually transmitted mite experienced an 80–100% reduction in egg viability. Similar reductions in fertility occurred in female noctuid moths, Spodoptera frugiperda, infected with a sexually transmitted nematode (Simmons and Rogers, 1994). To our knowledge this is the first empirical study on the effects of an insect STD on the reproductive physiology of a male host.
Here we examine the effects of a sexually transmitted nematode, Mehdinema alii (Nematoda: Diplogasterida), on the decorated cricket, Gryllodes sigillatus (Orthoptera: Gryllidae). Upon mating, female decorated crickets receive a two-part spermatophore consisting of a nutrient-rich spermatophylax and a sperm-containing ampulla. The ampulla is removed after the spermatophylax is consumed. Spermatophylax consumption can potentially thwart early removal of the ampulla by the female, which would interfere with sperm transfer. Thus, the spermatophylax serves primarily as a mating investment in this species (Sakaluk, 1984).
The nematode M. alii is a sexually transmitted endoparasite of the decorated cricket. The propagative stages of the nematode occur in the hindgut of adult male crickets only. This nematode is ovoviviparous and produces infective juveniles called dauer-larvae (dauers). The dauers reside in the genital chamber of sexually mature crickets where they are transferred during copulation. Once the dauers enter the male genital chamber, they invade the hindgut and develop into the parasitic, adult stage. Dauers transferred to the female cricket do not undergo further development; thus, adult females serve only as vectors for nematode transmission (Luong et al., 2000).
Initial observations suggest that the nematode does not cause high host mortality. This observation is consistent with the theory that STDs tend to evolve towards reduced virulence (Ewald, 1994; Lockhart et al., 1996). However, the evolution of spermatophylax production may be subject to strong parasite-host conflict. Because this parasite is transmitted during host copulation, it may benefit from a decrease in host spermatophylax size. If the nematode infection decreases a male cricket's ability to produce a sufficiently large or nutritious spermatophylax, then his mate may copulate with additional males, increasing the rate of nematode transmission. Yet, a suboptimal spermatophylax can be disadvantageous to the male because courtship feeding in insects can influence insemination success, the proportion of offspring sired, and overall reproductive success. We predict that under the pressure of parasitism, males infected with the nematode will produce a smaller spermatophylax than uninfected males.
An increase in the rate of contact between host and vector can potentially enhance the transmission rate of a sexually transmitted parasite. This can occur if the male intercopulatory interval is shortened. We expect the spermatophore replacement time to be shorter for an infected male compared to an uninfected male, either as a result of host pathology or behavioral manipulation by the parasite to enhance transmission. These two predictions are not necessarily mutually exclusive, as the physiological cost of producing a spermatophore may impose a trade-off between size and replacement time. Furthermore, while an increase in spermatophylax replacement rate would require elevated resource allocation to reproduction, presumably difficult under the physiological stress of parasitism, a decreased spermatophylax size should reduce resource requirements for reproductive activity.
Here we report on the effects of a sexually transmitted parasite on spermatophylax production in the decorated cricket. Spermatophylax mass was measured for healthy and parasitized males in two separate experiments. In the first experiment, males were infected by manually inoculating individuals with dauer stages of the nematode. In the second experiment, males were parasitized by allowing them to mate with infected females. This latter method of infection was also employed to parasitize a third set of males to determine the effects of the nematode on spermatophore replacement time.
METHODS
Decorated crickets, G. sigillatus, were collected from various sites on the campus of the University of California, Davis (USA). Initial dissections suggested that at least 50% of the males are infected (cf. 70%, Luong et al., 2000). Uninfected crickets were reared from the original colony by providing females with oviposition sites (moist cotton in petri dishes). Cricket nymphs were reared separately from adults to ensure nematode-free conditions. Late instars were isolated into same sex groups prior to the imaginal molt to ensure virginity. All crickets were maintained in an incubator at 28 ± 1°C, 30% relative humidity, and a 12-h light period. Crickets were provided with water and cat food (Purina Cat Chow™) ad libitum. Crickets were dissected at the end of each trial in 0.1 M NaCl under a dissecting microscope to assess the presence or absence and intensity of nematode infection.
Spermatophylax weight (manual infection)
To establish an infection in the experimental crickets, dauer stages of the nematode were extracted from the genital chambers of wild-caught female crickets (dissected in 0.1 M NaCl) and transferred to naive (virgin, nematode-free) male crickets, approximately 2–3 weeks post-eclosion. Ten to 20 dauers per male were transferred directly from the saline solution to the male rectum using an applicator stick with a human eyelash mounted at the tip. Control males were handled in a similar manner, except that the applicator stick was dipped into a nematode-free saline solution for a “blank” inoculation. All subjects were provided with food and water ad libitum.
Gryllids produce spermatophores independent of courtship and mating, and females need not be present to stimulate spermatophore production. Ten to 12 days post-inoculation, the spermatophylax was gently removed from the male's spermatophoric pouch (Alexander and Otte, 1967) using soft-tipped forceps and was transferred to a small piece of tared weighing paper. The male was weighed in a small plastic bag; the bag alone was reweighed afterwards. Both the cricket and the spermatophylax were weighed with a Mettler AE50 balance with an accuracy of ±0.2 mg. Control and infected males were dissected immediately to count the number of adult nematodes in their gut.
Spermatophylax weight (natural infection)
Infected males were established via the natural mode of infection, i.e., by mating with infected females. This method yielded an intensity of infection that more closely resembled infections observed in the field. For the treatment, one naive male was housed with three adult females and three adult males collected from the field (day 0). Because field-caught crickets are likely to be infected with the nematode, this treatment allowed nematode transmission to take place under semi-natural conditions. Preliminary experiments, in which only field-caught females were housed with the experimental male, yielded low infection rates. This is probably because the occurrence of dauers, in any given wild female, is ephemeral; hence, the likelihood of randomly selecting a female “loaded” with nematodes is low. Control males were established in a similar manner except that the experimental male was housed with nematode-free, non-virgin males and females. Both control and treatment males were marked with spots of paint (Paper-Mate™) on the pronotum for identification.
On day 7, all non-experimental males were removed so that any remaining dauers in the females would be transmitted to the experimental male. All females were removed one day prior to data collection to ensure the presence of a spermatophore. On day 10–12, 4 h into the dark photoperiod, the spermatophylax was extracted and both cricket and spermatophylax were weighed, as described above. Both control and infected males were dissected for nematodes.
While some authors correct for body size in reporting spermatophylax weight, we opted not to do so for the following reasons: (1) correcting for body size (measuring spermatophylax as percent body weight) yielded the same conclusions as uncorrected data; (2) post hoc analysis showed no significant correlation between body weight and spermatophylax weight for males in the control group; and (3) if parasitism depresses body weight, measuring spermatophylax weight as a percentage of male body weight may mask the effects of parasitism.
Spermatophylax replacement time
Second sets of treatment (n = 38) and control (n = 33) males were established via the natural mode of infections as described above. Males were isolated from females 2–3 days prior to the start of the mating observations to ensure that the males had sufficient time to generate a spermatophore.
Mating observations began on day 14, 4 h into the dark photoperiod. An experimental male was placed in an observation chamber with two virgin females (∼1–2 weeks post-adult eclosion) and provided with food and water. After the first mating, males were checked for the presence of a new spermatophore every 10 min for the next 8 h (male G. sigillatus in the field cease calling at about dawn; Sakaluk, 1987). The time elapsed between the first mating and the completion of a newly formed spermatophore was recorded as the spermatophylax replacement time.
RESULTS
Spermatophylax weight (manual infection)
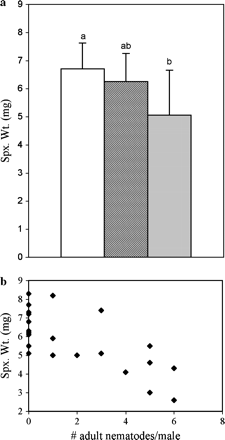
The manual infections yielded levels of infection that ranged from one to six adult nematodes per male (mean ± SD = 3.6 ± 2.0) in the treatment group. Control males (n =12) were nematode-free. Treatment males that became parasitized (‘infected-treatment,’ n = 12) produced a significantly smaller spermatophylax than did control males inoculated with a “blank” (mean ± SD for control males = 6.7 ± 0.9 mg, for infected males = 5.1 ± 1.6 mg; ANOVA, F1,22 = 9.6, p = .005). Many of the males to which we applied nematodes did not harbor any nematodes in their guts (‘uninfected-treatment’) when they were dissected (10 out of 24; 42%); they produced spermatophylaces of mean weight 6.3 ± 1.0 mg. When these males were included in the ‘treatment’ group, there was still a significant difference between the control and the treatment group (F1,32 = 5.62, p = .024). Post hoc analysis showed that the mean spermatophylax weight for uninfected-treatment males was not statistically different from either the infected or the control males (Tukey's mean comparison; Figure 1a). Spermatophylax weight was negatively correlated with the intensity of infection (R2 = .53, F1,22 = 24.9, p = .001; Figure 1b). Infected-treatment males with a heavier parasite burden produced smaller spermatophylaces than those males with few nematodes.
Results of manual infection experiment. (a) Mean spermatophylax weight (spx. wt.), error bars represent standard deviation. The mean spermatophylax weight was significantly higher for control males (white bar, n = 12) than for infected males (light bar, n = 12) (p = .005). The mean wpermatophylax weight for uninfected-treatment males (dark bar, n = 10) did not differ from that of control or infected-treatment males. (b) Spermatophylax weight is negatively correlated with the number of adult nematodes (intensity of infection; R2 = .53, p = .001). Each data point represents an individual male cricket. Spermatophylax weights for control males are also included in graph (i.e., intensity of infection = 0). The mean parasite load was 3.6 nematodes/male.
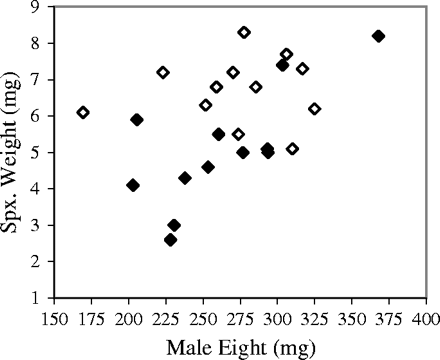
There was no significant correlation between male body weight and spermatophylax weight for control males (R2 = .003, F1,10 = 0.03, p = .86). However, there was a significant positive correlation between male body weight and spermatophylax weight for infected males (R2 = .53, F1,10 = 11.3, p = .007), such that smaller males produced smaller spermatophylaces (Figure 2). The mean male body weight did not differ between control (262.7 ± 41.4 mg), uninfected-treatment (273.5 ± 49.0 mg), and infected-treatment males (245.4 ± 36.1 mg; F2,31 = 0.27, p = .77).
Results from manual-inoculation experiments show a positive correlation between male weight and absolute spermatophylax weight. This relationship was statistically significant only for infected male crickets (white: control n = 12, R2 = .003, p = .86; black: infected n = 12, R2 = .53, p = .007).
Spermatophylax weight (natural infection)
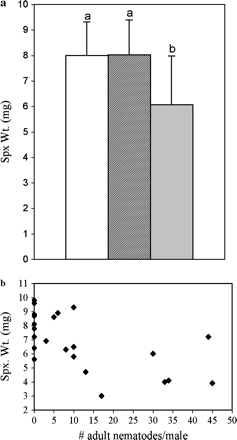
The intensity of infection in the treatment group ranged from 3 to 45 nematodes per male cricket (mean ± SD = 18.5 ± 14.5). None of the males in the control group (n = 14) harbored nematodes. Infected-treatment males (n = 15) produced a significantly smaller spermatophylax than control males (mean ± SD for control males = 8.0 ± 1.3 mg, for infected-treatment males = 6.1 ± 1.9 mg; F1,27 = 12.8, p = .004). When we included both the infected and uninfected males (n = 8) in the treatment group, we still detected a significant difference between the control and the treatment group as a whole (F1,35 = 4.40, p = .043). Post hoc analysis showed that the mean spermatophylax weight was significantly different between uninfected- and infected-treatment males, but not between control and uninfected-treatment males (uninfected = 8.0 ± 1.4 mg, Tukey's mean comparison; Figure 3a). Also, there was a negative correlation between parasite burden and spermatophylax weight (Figure 3b; R2 = .37, F1,27 = 15.5, p < .001).
In the natural infection experiment, (a) mean (+SD) spermatophylax weight (spx. wt.) for control males (white bar, n = 14) is significantly higher than for infected males (light bar, n = 15) (p < .004). The mean spermatophylax weight for uninfected-treatment males (dark bar, n = 8) did not differ from that of control males. (b) The negative correlation between parasite load and spermatophylax weight is significant (R2 = .37, p < .001). Each data point represents an individual cricket, either a control (intensity of infection = 0) or an infected male. The mean parasite load was 18.5 nematodes/male.
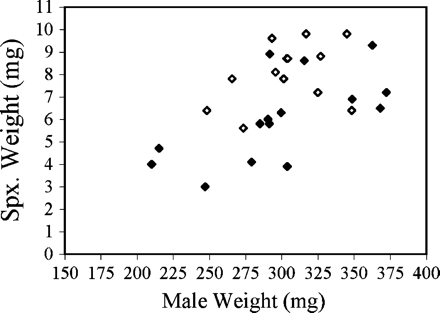
The correlation between male body weight and spermatophylax weight was not significant for control males (R2 = .11, F1,12 = 1.43, p = .25). However, there was a positive correlation between male body weight and spermatophylax weight for infected males (R2 = .42, F1,13 = 9.4, p = .009). Again, the negative impact of the nematode infection on spermatophylax weight was more apparent for smaller males than larger males (Figure 4). The mean body weights for control (305.2 ± 28.9 mg), uninfected (318.6 ± 41.4 mg), and infected males (298.8 ± 50.3 mg) were not significantly different (F2,34 = 0.60, p = .55).
Results from natural-infection experiments show a positive correlation between male weight and absolute spermatophylax weight. This relationship was significant only for infected male crickets (white: control n = 14, R2 = .11, p = .25; black: infected n = 15, R2 = .42, p = .009). Note that two data points (control) are partially obscured.
Spermatophylax replacement time
Of the 38 treatment males, all but six acquired the nematode infection. Of the 32 infected males, 29 produced a second spermatophore within the 8-h observation period. The intensity of infection ranged from one to 150 adult nematodes in the gut (mean ± SD = 19.7 ± 29.2). None of the 33 control males harbored nematodes, and all but seven males replaced the spermatophore within the observation period. The mean spermatophore replacement times for control and infected males were not significantly different (mean for control, 173 ± 115 min; mean for infected, 179.8 ± 129.8 min; Wilcoxon sum-rank test, control n = 26, infected n = 29, p > .1). No signs of morbidity or mortality were observed in either the control or treatment groups during this experiment.
DISCUSSION
Experiments employing manual and natural infections both show that parasitism reduces the weight of the spermatophylax produced by infected males. The negative correlation between parasite load and spermatophylax weight strongly suggests that the parasite exerts a dose-dependent, negative effect on spermatophylax formation. Moreover, when the treatment group included both uninfected and infected males, the statistical difference in spermatophylax weight between the control and treatment group persisted. This somewhat conservative analysis verifies that spermatophylax production among infected males is adversely affected by parasitism and that the infected males do not simply represent a sample of low quality males.
In some cases the treatment males failed to acquire the nematode infection. The failure may be due to several reasons: (1) dauer nematodes were damaged or lost during manual inoculations; (2) males in natural infections failed to mate with an infected female; and/or (3) males acquired the infection, but lost it prior to data collection.
Parasite-mediated reduction in host spermatophore formation was also reported in field crickets, Gryllus veletis and G. pennsylvanicus, where the number of spermatophores produced was negatively correlated with natural levels of a gregarine infection (Zuk, 1987). Simmons (1993) also showed that male bush crickets (Orthoptera: Tettigoniidae) experimentally infected with gregarines had reduced spermatophore production compared to uninfected males.
Although there was a fivefold difference in the intensity of infection across the two experiments (manual 3.6, natural 18.5), the mean spermatophylax size for infected males in the natural-infection experiment (6.1 mg) was actually larger than that of males in the manual-infection experiment (5.1 mg). Males in the manual-infection experiment (245 mg) were on average smaller than the males in the natural-infection experiment (298 mg), and because spermatophylax size is influenced by body size this may explain the discrepancy. Also, these two experiments were conducted under different conditions: with varying modes of infection, using a separate batch of males, and at different times. So, while the results are quantitatively different, they are qualitatively similar in that they both demonstrate an adverse effect of the nematode on host spermatophylax investment.
Interestingly, the positive correlation between male body weight and spermatophylax weight in our study was only significant for infected and not for control males (both manual and natural infections). A test for homogeneity of slopes (the null hypothesis) showed no interaction between male body weight and treatment effect (p = .54). Our inability to detect a significant correlation in the control group may be due to the relatively small range in body weights in this experiment. The association between body weight and spermatophylax size among parasitized males may be due to a parasite-induced decline in total body weight. However, this scenario is unlikely because the mean body weight did not differ significantly between control and infected males (manual and natural infections). Future studies should address the relationship between parasitism, spermatophylax weight, and pre-infection male mass.
Although parasitism suppressed spermatophylax size, the spermatophylax replacement time was not significantly different between control and infected males. Spermatophore synthesis is costly (Sakaluk, 1985), and there may be strong physiological constraints against increasing the rate at which spermatophores are replaced. Furthermore, there may be an upper limit to the benefits accrued to the nematode from increasing male mating frequency. Because the nematode, M. alii, is dioecious, an increase in male host mating frequency could result in nematodes moving to hosts in clutches that are too small to successfully initiate a breeding population. In other words, if the initial nematode density is below some required threshold, an Allee effect (Deredec and Courchamp, 2003; Regoes et al., 2002) may result.
Our inability to detect a difference in spermatophylax replacement time may be the result of a potentially small effect size and/or high variance in the data. However, the difference revealed in the experiment is the opposite of what we predicted. So, while we were unable to detect a significant difference in spermatophylax replacement time between control and infected males, there is no evidence that the small spermatophylaces produced by infected males allowed for faster replacement time.
In this experiment the mortality rate did not differ significantly between the control and treatment groups. This finding is consistent with the theory that sexually transmitted parasites are expected to evolve reduced virulence (Ewald, 1994; Lockhart et al., 1996).
Although the exact mechanism by which the nematode suppressed host spermatophylax investment is unknown, it is likely that the presence of adult nematodes in the hindgut interferes with water reabsorption or competes with the host directly for nutrients. Given that the synthesis of nuptial gifts is costly, any nutritional or other physiological stress imposed by the parasite could limit the male's ability to invest optimally in spermatophore production. Therefore, the observed reduction in spermatophylax size among parasitized males may be a direct consequence of host pathology.
The decline in spermatophylax size could potentially result in a reduction in ampulla attachment time (Sakaluk, 1984, 1985). Males that produce a small spermatophylax may be penalized by the early removal of the ampulla by the female (Sakaluk, 1984). Therefore, male insemination success may suffer if he is unable to provision his mate with a sufficiently large spermatophylax. This may also serve as mechanism for “cryptic” female choice (Thornhill, 1983), in which small, infected males are at a selective disadvantage.
However, a parasite-host conflict arises, in that selection via parasite evolution should favor a reduction in spermatophylax weight. If early ampulla removal truncates the time for sperm transfer, this may lead to a decrease in female intercopulatory interval (Gwynne, 1986; Simmons and Gwynne, 1991). Lehmann and Lehmann (2000a,b) found that in bush crickets parasitized with parasitoids, females that mated with parasitized males received a relatively smaller spermatophylax, and this resulted in shorter intercopulatory intervals compared to females that mated with unparasitized males. Because the parasite in our study is sexually transmitted and its reproductive success is directly linked to host mating success, an increase in host mating activity can potentially lead to an increase in the rate of nematode transmission (Lockhart et al., 1996).
Therefore, an alternative explanation for the reduction in spermatophylax size is that an increase in sexual activity, via alterations in host behavior or induced sterility, can potentially result in repeated mating attempts by the female, and hence increased parasite transmission. Further studies are needed to determine whether the parasite-mediated reduction in spermatophylax weight actually translates into changes in female mating behavior and parasite transmission rate. If this is the case, parasite-induced alterations in host spermatophylax production may be attributed to parasite manipulation (Beckage, 2002; Moore and Gotelli, 1996).
We thank Ann Hedrick, Jay Rosenheim, and two anonymous referees for their comments. Also, thanks to Hien Trinh for her assistance in the care and maintenance of the cricket colony. Partial funding was provided by a Jastro-Shields Research Grant and by the Genetics Resources Conservation Program at the University of California, Davis.
References
Alexander RD, and Otte D,
Beckage NE,
Brown JJ, and Reed DA,
Carver FJ, and Hurd H,
Deredec A, and Courchamp, F,
Gwynne DT,
Hurd H,
Hurst GDD, Sharpe RG, Broomfield AH, Walker LE, Majerus TMO, Zakharov IA, and Majerus MEN,
Knell RJ,
Lehmann GUC, and Lehmann AW,
Lehmann GUC, and Lehmann AW,
Lockhart AB, Thrall PH, and Antonovics J,
Luong LT, Platzer EG, Zuk M, and Giblin-Davis RM,
Moore J, and Gotelli, NJ,
Regoes RR, Ebert D, and Bonhoeffer S,
Sakaluk SK,
Sakaluk SK,
Sakaluk SK,
Sheldon BC,
Simmons LW,
Simmons LW, and Gwynne DT,
Simmons AM, and Rogers CE,
Thornhill R,
Thrall PH, Antonovics J, and Bever JD,
Author notes
aDepartment of Entomology
bDepartment of Nematology, University of California, Davis