Abstract
We are starting to understand the relationship between metabolic rate responses and species’ ability to respond to exposure to high pCO2. However, most of our knowledge has come from investigations of single species. The examination of metabolic responses of closely related species with differing distributions around natural elevated CO2 areas may be useful to inform our understanding of their adaptive significance. Furthermore, little is known about the physiological responses of marine invertebrate juveniles to high pCO2, despite the fact they are known to be sensitive to other stressors, often acting as bottlenecks for future species success. We conducted an in situ transplant experiment using juveniles of isopods found living inside and around a high pCO2 vent (Ischia, Italy): the CO2 ‘tolerant’ Dynamene bifida and ‘sensitive’ Cymodoce truncata and Dynamene torelliae. This allowed us to test for any generality of the hypothesis that pCO2 sensitive marine invertebrates may be those that experience trade-offs between energy metabolism and cellular homoeostasis under high pCO2 conditions. Both sensitive species were able to maintain their energy metabolism under high pCO2 conditions, but in C. truncata this may occur at the expense of [carbonic anhydrase], confirming our hypothesis. By comparison, the tolerant D. bifida appeared metabolically well adapted to high pCO2, being able to upregulate ATP production without recourse to anaerobiosis. These isopods are important keystone species; however, given they differ in their metabolic responses to future pCO2, shifts in the structure of the marine ecosystems they inhabit may be expected under future ocean acidification conditions.
Similar content being viewed by others
Introduction
As a result of the ongoing anthropogenic increase in CO2 emissions, the level of seawater pCO2 is expected to increase, resulting in a decrease in oceanic pH by 0.4–0.5 pH units by the year 2100 (Caldeira and Wickett 2003; IPCC 2014); a phenomenon known as ocean acidification (OA). However, despite a plethora of recent studies, we still know relatively little about the homoeostatic metabolic responses of marine ectotherms to high pCO2 conditions compared to other climate-driven parameters such as temperature (Somero 2005; Pörtner 2008; Gunderson and Stillman 2015; c.f. Stillman and Paganini 2015). Most previous investigations have been single species orientated and taxonomically restricted, mainly to heavily calcified marine invertebrate species (e.g. corals, sea urchins and molluscs), which have been predicted to experience the greatest negative impacts from any decrease in oceanic pH due to calcium carbonate dissolution, being adversely affected by changes in ocean chemistry (Fabry et al. 2008; Wittmann and Pörtner 2013). The fact that shell biomineralisation processes would likely need to be upregulated in low pH conditions can arguably have a significant impact on an organism’s energy metabolism, with changes predicted to occur in the direction and the intensity of the metabolic response (e.g. Pörtner 2008; Lannig et al. 2010; Melatunan et al. 2011; Maas et al. 2012; Ivanina et al. 2013; Melatunan et al. 2013; Klok et al. 2014). Such changes would likely lead to a trade-off between an organism’s capacity to maintain or increase its metabolic rates and other fundamental, energy demanding, physiological processes, such as growth and reproduction. This would ultimately be most detrimental to an organism’s overall fitness (Saba et al. 2012; Edmunds et al. 2013; Rivest and Hofmann 2014; Uthicke et al. 2014).
In comparison, crustaceans, including juvenile and larval stages have been shown to be relatively tolerant to decreases in seawater pH predicted to occur for the end of the century (Widdicombe and Spicer 2008; Melzner et al. 2009; Ries et al. 2009; Ross et al. 2011; Whiteley 2011; Branch et al. 2012; Arnberg et al. 2013; c.f. Walther et al. 2011; Small et al. 2015). This is probably due to the fact that many crustacean species are characterised by fairly low degrees of calcification, but are also able to successfully regulate internal acid–base balance, and whilst this necessitates an increase in metabolic effort, overall homoeostasis appears to remain intact (Spicer et al. 2007; Small et al. 2010; Rastrick et al. 2014). However, within crustaceans, a number of groups remain understudied in terms of the effect of pCO2 levels projected to occur by the end of the century (Caldeira and Wickett 2003; IPCC 2014) on the metabolic costs of maintaining homoeostasis. One such group is the marine isopods which are considered to be important keystone species in marine ecosystems, including in shallow water seagrass habitats (see review by Poore and Bruce 2012). The isopod cuticle contains magnesium calcite, which is highly soluble (Andersson et al. 2008; Neues and Epple 2008), and as a result isopods are potentially at risk of being negatively affected by OA conditions (Alenius and Munguia 2012; Jakubowska et al. 2013; Munguia and Alenius 2013; Wood et al. 2014). Furthermore, very few studies examining the effects of OA on fundamental physiological processes have focussed on juvenile (post-larval) stages of lower calcifying marine invertebrates (i.e. those with lighter, less mineralised skeletons), with only a handful of studies published to date (e.g. Carter et al. 2013; Ceballos-Osuna et al. 2013; Knapp et al. 2015; Small et al. 2015; Zheng et al. 2015), despite juveniles being considered as sensitive bottlenecks for population recruitment, including in crustaceans (Walther 2010; Small 2013). Instead, where impacts of OA on early life stages have been investigated, the vast majority of these studies have been restricted to measurements of shell dissolution rates and/or ecological and life history traits such as growth, survivorship and/or settlement in heavily calcified species (Melzner et al. 2009; Byrne 2011; Ross et al. 2011; Whiteley 2011). It is therefore evident that, overall, juveniles have been overlooked, partly as a result of often being seen as identical, but simply morphometrically smaller versions of adult species (Page 2009). During the juvenile phase, many significant physiological and behaviour changes take place, including metamorphosis and organogenesis, onset of sexual maturity and changes in diet and habitat (Hadfield 2000; Byrne 2011; Ross et al. 2011). Consequently, recent work has suggested that juveniles may, in fact, be particularly physiologically sensitive, therefore acting as bottlenecks for the longevity of individual species, especially under ongoing global change scenarios (Walther et al. 2010; Byrne 2011; Walther et al. 2011). It is surprising therefore that quantifying the physiological response to OA in juveniles of marine invertebrates has not attracted further interest.
Shallow water high CO2 vents have been used as analogues to investigate the potential ecological and evolutionary implications of OA (e.g. Hall-Spencer et al. 2008; Kroeker et al. 2011; Rodolfo-Metalpa et al. 2011; Calosi et al. 2013a, b; Lucey et al. 2015; Ricevuto et al. 2015; Gambi et al. 2016). At the high pCO2 vent of the Castello Aragonese at Ischia (Italy), the invertebrate fauna have been characterised along the existing CO2 gradient. This includes assemblages comprising juveniles of several isopod species with different distribution patterns, seemingly as a result of their tolerance or sensitivity to high pCO2 conditions (Kroeker et al. 2011; Ricevuto et al. 2012). Based on their distribution, marine invertebrates found inside and outside these naturally acidified areas have been defined as either ‘tolerant’ (abundant inside and often outside the low pH/high pCO2 areas) or ‘sensitive’ (found only outside the vents in similar habitat) (Calosi et al. 2013b; Turner et al. 2015). Recent work on an annelid assemblage at this site has shown clear differences between the metabolic responses of closely related species that overlap in their distribution patterns. Those species classed as ‘tolerant’ are able to maintain their metabolic rate levels during acute exposure to elevated pCO2, whereas those classed as ‘sensitive’ are not, and, instead show significant up or downregulation of metabolic rate (Calosi et al. 2013b). To some extent, these patterns of relationships between species’ distribution and metabolic rate responses to pCO2 appear to be consistent across multiple natural systems characterised by pCO2 gradients such as those explored by Maas et al. (2012) and Lewis et al. (2013). However, recent work has suggested that marine invertebrates classed as pCO2 ‘sensitive’ may be those that experience trade-offs between energy metabolism and cellular homoeostasis under high pCO2 conditions. Homoeostatic capacity would be expected to be higher in ‘tolerant’ species, and thus they would not be expected to experience this trade-off (Turner et al. 2015).
In order to test the hypothesis that trade-offs between energy metabolism and cellular homoeostasis characterises the response of ‘sensitive’ species to high pCO2 conditions, as well as to examine the level of physiological adaptation or physiological plasticity to high pCO2 in the juvenile stages of a marine invertebrate assemblage, we carried out a series of in situ transplant experiments. These utilised juveniles of three species living inside and around the high pCO2 vent site of Castello Aragonese (Ischia, Naples, Italy). More specifically, we investigated the metabolic responses of the CO2 ‘tolerant’ Dynamene bifida Torelli, 1930 and CO2 ‘sensitive’ Cymodoce truncata (Leach, 1814) and Dynamene torelliae Holdich, 1968; ‘tolerant’ and ‘sensitive’ being defined by these species’ residence either inside or outside the vents, respectively (Kroeker et al. 2011; Ricevuto et al. 2012). Little is known about the effects of high pCO2 on early life stages of Peracarida, with only a few studies to date (Egilsdottir et al. 2009; Hauton et al. 2009). Juveniles of these three species are found on hard bottoms and also within Posidonia oceanica meadows and seaweed dominated habitats which at Ischia occur in both high and low pCO2 areas inside and outside the vent, respectively (Cigliano et al. 2010; Ricevuto et al. 2012). Our experimental design allowed us to compare and contrast the strategies used (i.e. degree of metabolic adaptation or metabolic acclimatisation) by this understudied, but ecologically important group of marine invertebrates with what we know about the annelid fauna found in the same area (Calosi et al. 2013b; Turner et al. 2015). The results obtained will allow us to unravel possible functional trade-offs among different traits and across different taxonomic groups, which may help explain the potential physiological sensitivity or the physiological tolerance of each of these species to high pCO2. After exposure to either low or high pCO2, we examined the concentration levels of fundamental aerobic and anaerobic metabolites (i.e. ATP, l-lactate, respectively) and those of carbonic anhydrase, an essential enzyme involved in an organism’s acid–base and respiratory function (Henry 1996) in each and every experimental individual (Bennett 1987; Calosi et al. 2013c; Turner et al. 2015). The use of an individual approach where we rigorously compared the levels of these three key metabolites allowed us to thoroughly test our hypothesis that marine invertebrates classed as pCO2 ‘sensitive’ may be those that experience trade-offs between energy metabolism and cellular homoeostasis under high pCO2 conditions.
Materials and methods
Animal collection and preparation for in situ transplant
All juvenile isopods were collected via SCUBA and snorkelling between June and September 2013. Juveniles of both Dynamene and Cymodoce are morphologically distinct from adults. Adult males are distinguished by the presence of a fully developed peraeonal bidentate process (Dynamene) or prominent pleonal tubercles (Cymodoce). Adult females of both genera feature modified mouthparts. Moreover, there is a sharp habitat separation between adults and juveniles of all three species used in this study. Adults (non-feeding) live in crevices, whereas juveniles (actively feeding) are found on seaweeds and seagrasses (M. Lorenti, personal observation).
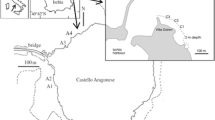
Juveniles of the two ‘sensitive’ species C. truncata (n = 60, mean 13.07 ± 1.63 mg) and D. torelliae (n = 73, mean weight 3.06 ± 0.19 mg) were collected at two control sites (low pCO2/high pH) (C) at 1–2 m depth off St. Anna’s rocks, Ischia (Naples, Italy) (40°43′34″N, 13°57′35″E) approx. 600 m from the Castello south side vents, and at San Pietro promontory, Ischia, approximately 4 km from the venting site (40°44′48″N, 13°56′39″E) where pH values are representative of low pCO2 conditions (mean pH 8.13 ± 0.01) (Calosi et al. 2013b; Ricevuto et al. 2015), whilst juveniles of D. bifida (n = 90, mean weight 2.74 ± 0.21 mg) were collected at an acidified site (high pCO2/low pH) (A) 1–2 m depth on a rocky reef in an area with acidified conditions and high CO2 venting activity (>10 vents m2) on the south side of the Castello Aragonese, Ischia (40°43′53″N, 13°57′47″E) (mean pH 7.29 ± 0.04) (stations S2/S3 in previous studies, e.g. Cigliano et al. (2010)). For a detailed description of the collection and study areas see Calosi et al. (2013b). All specimens were transferred to the Villa Dohrn—Benthic Ecology Centre (approx. 4 km from the vents) within 30 min of collection using cool boxes filled with seawater of the appropriate pH from the collection site (approx. vol. = 10 L). The ‘cool box:isopod’ volume ensured that changes in temperature, salinity, O2 and pH were minimised during transport. Once in the laboratory, isopods were sorted from macroalgae by shaking the thalli inside plastic trashes, and maintained for 2 days prior to the experiment in glass bowls (approx. 20 indiv. per bowl), each containing 300 mL of natural seawater at the original pH/pCO2 which was changed daily. All glass bowls were kept in a temperature control room (T = 19 °C, 12 L:12 D cycle). Isopods were fed daily with fragments of macroalgae (mostly Cladophora, Dictyota and Halopteris) collected at the respective sampling sites.
Experimental design, study area and experimental procedure
In order to characterise the presence of potential physiological mechanisms underpinning the apparent differences in sensitivity to high pCO2 of the three species of isopods investigated, as evidenced by their differential distribution around the CO2 vent, an in situ transplant experiment utilising the natural CO2 vents of Ischia was conducted. Isopods are highly motile compared to many other benthic marine invertebrate species. However, the juveniles collected for this study were all collected from macroalgae growing on rocky substrate and P. oceanica dead mat. Cymodoce truncata and D. torelliae collected from control areas (C) and were transplanted both to another control area (at the S. Pietro promontory) (e.g. CC) and to the acidified (vented) area (e.g. CA). In the case of D. bifida, individuals were collected from the acidified area (A) and transplanted to the control area (e.g. AC) and also maintained in the acidified area (e.g. AA). In each area, three stations were identified, approx. 50 m from each other and designated as C1, C2 and C3 for control, and A1, A2 and A3 for the high pCO2 vented area (c.f. Calosi et al. 2013b), in order to allow for spatial replication. Each station consisted of a weighted line with a buoy (approx. 2.5 m depth) to which the experimental containers or ‘transplantation chambers’ (TCs) could be attached. These were constructed from white PVC tubes (diameter = 4 cm, length = 11 cm) with a nytal plankton net (mesh = 100 µm) fixed to both ends. This net size allowed for the continual flow through of seawater, but at the same time prevented the isopods from escaping, being washed away or being predated upon.
On the day of deployment, isopods were transferred to the TCs (max. 15 indiv. per TC) underwater. These were then transferred to tanks (approx. vol. 10 L each, three TCs per tank) and transported to the experimental area: directly from land for the control area and via boat in the case of the acidified area. TCs were then immediately deployed by SCUBA to each station (n = 1 TC per station) in both the control and acidified areas where they remained for 5 days under natural conditions, according to the experimental design by Calosi et al. (2013b) and Turner et al. (2015). Seawater temperature, salinity and pH were measured at each station at the same time each day during the 5 days experimental period, and diel flux was minimal in the abiotic parameters measured (Lucey 2016; Lucey et al. 2016). Seawater samples were also taken for total alkalinity analyses (see Suppl. Mat.).
After 5 days exposure, TCs were recovered by SCUBA, placed underwater (in order to avoid any exposure to air) in 10-L tanks containing fresh seawater of the appropriate pH collected from the respective experimental areas. To minimise environmental shocks, isopods were transported to the laboratory within 30 min. Upon arrival in the laboratory, isopods were rapidly and carefully removed from the TCs, immediately weighed and snap frozen in liquid nitrogen inside 1.5-mL screw cap microcentrifuge tubes before being shipped to the Marine Biology and Ecology Research Centre (MBERC) at Plymouth University on dry ice. At MBERC, the concentration levels of fundamental aerobic and anaerobic metabolites were determined in each and every experimental individual (Bennett 1987; Calosi et al. 2013c; Turner et al. 2015). The use of an individual approach where we rigorously compared the levels of these three key metabolites allowed us to thoroughly test our hypothesis that marine invertebrates classed as pCO2 ‘sensitive’ may be those that experience trade-offs between energy metabolism and cellular homoeostasis under high pCO2 conditions. We decided to focus on two of the most fundamental indicators of metabolism: ATP and l-lactate. ATP is the primary source of free energy in all living cells, produced by aerobic metabolism, whereas l-lactate is the main indicator of anaerobic metabolism in crustaceans and thus can be indicative of metabolic stress (Urich 1994). We also measured levels of carbonic anhydrase, an essential enzyme involved in an organism’s acid–base and respiratory function (Henry 1996). Levels of this enzyme can also inform on biomineralisation processes, including in low-calcifying groups including Peracarida (Meyran et al. 1987). Concentrations of each of these were examined in each individual tested after exposure to either low or high pCO2. We hypothesised that when exposed to high pCO2 conditions, ‘sensitive’ species will upregulate their metabolic machinery to maintain homoeostasis (Calosi et al. 2013b) causing an associated increase in ATP (Turner et al. 2015). If these species have resorted to anaerobic respiration to maintain ATP production, then l-lactate concentrations will also increase. These ‘sensitive’ species would also need to upregulate acid–base and respiratory function upon exposure to high pCO2 conditions; therefore, levels of carbonic anhydrase would also be likely to increase. ‘Tolerant’ species exposed to low pCO2 conditions would likely experience less demand on their acid–base and metabolic function resulting in lower levels of ATP, l-lactate and carbonic anhydrase, the exact levels of which as well as the ratio between levels could indicate any metabolic trade-off response between different physiological processes as well as the degree of adaptation or acclimatisation of these species to high pCO2 conditions (Calosi et al. 2013b).
Biochemistry
At the MBERC laboratory, specimen levels of ATP, l-lactate and carbonic anhydrase were determined. Beforehand whole animal tissue extracts were prepared. All extraction steps were completed in a temperature controlled room at 4 °C. Individuals were removed from storage at −80 °C and homogenised by hand using chilled disposable plastic micropestles (Eppendorf, Eppendorf AG, Hamburg, Germany) in 1.5-mL microcentrifuge tubes containing ice-cold 5 % trichloroacetic acid (TCA) at a ratio of 16:1 (TCA µL: body mass mg). The homogenate was then centrifuged for 10 min at 17,000g at 4 °C. The resulting supernatant was then removed to a second ice-chilled 1.5-mL microcentrifuge tube and used to determine [ATP], [l-lactate] and [carbonic anhydrase].
Concentrations of l-lactate and carbonic anhydrase were determined spectrophotometrically in the isopod extracts. These assays were undertaken in a microplate format using a VERSAmax™ plate reader (Molecular Devices, Sunnyvale, CA, USA). l-lactate concentrations were assayed using a commercial kit (l-lactate 735, Trinity Biotech Wicklow, Ireland). CA concentrations were measured using the methods detailed in Ivanina et al. (2013) but adapted to a microplate format.
ATP concentrations were determined using a commercial luciferase-based kit (ATP Kit SL 144-041, BioThema, Handen, Sweden). The reaction between luciferase and luciferin in the presence of ATP produces light with the amount of light emitted being directly proportional to the amount of ATP present. Luminescence was measured using a luminometer (Pi-102, Hygiena LLC, Camarillo, CA, USA). The difference in the slope of the reaction with and without the internal ATP standard was used to determine sample ATP concentration.
Statistical analyses
GLM tests were used to investigate the effect of exposure to high or low pCO2 conditions found in the transplanted areas (inside or outside the CO2 vents, depending on species) on the mean cellular physiological traits investigated separately, with the term ‘station’ as a random factor nested within the pCO2 treatment and individual ‘body mass’ as a covariate. All data met assumptions for normality, although some data for carbonic anhydrase had to be log10 transformed beforehand (as indicated in Table 1) and for homogeneity of variances. In a preliminary analysis, both the terms ‘station’ and ‘body mass’ (within the range tested) were shown not to have a significant effect on the biological variables investigated and were therefore removed. To rigorously test our hypothesis whilst avoiding any limitation surrounding the use of the ‘golden mean’, individual approach analyses were also utilised (Bennett 1987), where individual values for the levels of the three key metabolites measured were correlated using the Pearson correlation test. All analyses were conducted in SPSS version 21.
Results
Survival rates were overall good and comparable across all species investigated at the different pCO2 treatments, with an overall average survival rate of 74 % (Table S1). Results for the carbonate system (Table S2) confirmed that pH and pCO2 differed significantly outside (C) and inside (A) the CO2 vent areas, consistent with previous studies (Kroeker et al. 2011; Calosi et al. 2013b; Ricevuto et al. 2015).
Following in situ transplantation inside TCs, the average survival rate of juvenile isopods was 74 %. Mean values for the biochemical parameters measured are given in Table 1. Exposure in situ of both the ‘sensitive’ C. trunctata and D. torelliae to high pCO2 did not exert a significant effect on [ATP] (Fig. 1a), whilst it leads to a significant decrease in mean [l-lactate] (Fig. 1b). In addition, [carbonic anhydrase] decreased significantly in C. truncata and increased significantly in D. torelliae (Fig. 1c) following exposure to high CO2. The ‘tolerant’ species D. bifida when transplanted from high pCO2 conditions to low pCO2 (AC) conditions showed a significant increase in [ATP] (Fig. 1a), and no significant difference in either [l-lactate] or [carbonic anhydrase] (Fig. 1b, c). Individual analyses revealed non-significant correlations between [ATP] and [l-lactate] and [ATP] and [carbonic anhydrase] for all three species examined. The exception was that of a positive relationship between [ATP] and [l-lactate] for the ‘sensitive’ D. torelliae exposed to low pCO2 (r = 0.482, P = 0.031). This relationship broke down when the isopods were exposed to high pCO2 conditions (r = 0.17, P = 0.561) (see Suppl. Mat.).
Reaction norms as contrast plots showing percentage change of mean a [ATP] (nmol mg−1), b [l-lactate] (nmol mg−1) and c [carbonic anhydrase (CA)] (nmol mg−1) in the isopods Cymodoce truncata [sensitive (S)], Dynamene torelliae [sensitive (S)] and Dynamene bifida [tolerant (T)] when isopods were exposed to high pCO2/low pH or low pCO2/high pH conditions in a transplanted environment either inside or outside the vented area. The sensitive Cymodoce truncata and Dynamene torelliae were collected from low pCO2/high pH conditions outside the vents (C) and transplanted to high pCO2/low pH conditions inside the vents (A) (e.g. CA). The tolerant Dynamene bifida were collected from high pCO2/low pH conditions within the vents (A) and transplanted to low pCO2/high pH conditions outside the vents (C) (e.g. AC). Mean biochemical parameter measured in original environment was set as 100 %, and mean biochemical parameter measured in transplanted environment recalculated accordingly. Dashed line indicates no change in reaction norm from 100 %. Asterisk indicates the presence of a significant difference between the mean biochemical parameter measured in the original environment and transplanted environment according to the GLM test (P < 0.05)
Discussion
In this study, we show the differing metabolic responses of three closely related marine isopods to high and low pCO2 conditions during an in situ transplant experiment at a natural shallow water high pCO2 vent system. Our data demonstrate that differences in the distribution of these three species in and around the Ischia high pCO2 vent can begin to be explained by either the physiological adaptation or plasticity (acclimatisation response) of each species to high pCO2 conditions. In a broader context, our findings strengthen the idea that the distribution of marine invertebrates to future high pCO2 conditions will be, at least in part, dictated by their ability to increase their metabolic and homoeostatic capacities (Melzner et al. 2009; Calosi et al. 2013b; De Wit et al. 2015; Magozzi and Calosi 2015; Turner et al. 2015).
For the two sensitive species C. truncata and D. torelliae, there was no change in ATP concentration when they were exposed to high pCO2 conditions, with this being accompanied by a decrease in l-lactate concentration. This suggests that despite being considered sensitive to high pCO2, both these species have a degree of capacity for metabolic plasticity, at least during our short-term experiment, enabling them to maintain a certain level of homoeostasis when facing high pCO2 conditions, e.g. ATP production appears to be maintained without recourse to anaerobic pathways (Urich 1994). Some species of marine isopods have been recorded as being metabolically tolerant to extreme environmental conditions. The Baltic isopod Saduria entomon which is closely related to Dynamene and Cymodoce is able to maintain aerobic metabolism during conditions of extreme hypoxia (Hagerman and Vismann 1997) and several other isopod species, e.g. Stenasellus virei are known to have a high capacity for glyconeogenesis from lactate and a faster and more complete replenishment rate of ATP and arginine phosphate after hypoxic exposure (Hervant et al. 1997). Saduria entomon also appears to be metabolically tolerant to OA conditions (Jakubowska et al. 2013), whereas others are negatively impacted in terms of their metabolic (Alenius and Munguia 2012) and immunological (Wood et al. 2014) homoeostatic capacity. Our results suggest that metabolic capabilities enabling species to tolerate extreme conditions may be more widespread throughout isopods than previously thought, although longer exposure experiments are required to allow further exploration of this hypothesis. However, even though our results indicate that both C. truncata and D. torelliae possess the metabolic capability to withstand the high pCO2 conditions inside the vented area, there may still be other, as yet unknown, ecological factors, e.g. low food supply and/or limitations on these species interactions with the dominant macroalgae (Kroeker et al. 2011) that prevent these species inhabiting this site.
However, for the two sensitive species (C. truncata and D. torelliae), there is a species-specific difference in the reaction norm for carbonic anhydrase after exposure to high pCO2 conditions. In the case of C. truncata, there is a significant decrease in carbonic anhydrase concentration under high pCO2 conditions, whereas for D. torelliae, there is a significant increase. This evidence, together with the fact that in both species ATP production is maintained under high pCO2 conditions indicates the presence of physiological trade-offs. The utilisation of physiological trade-offs allowing the maintenance of energy metabolism at the expense of cellular homoeostasis has recently been proposed for another high pCO2 sensitive marine ectotherm, the fan worm, Sabella spallanzanii (Turner et al. 2015). This species is also found around the high pCO2 vent at Ischia but never inside the CO2 venting areas, and when exposed to high pCO2 conditions has been shown to upregulate ATP production at the apparent cost of carbonic anhydrase concentration (Turner et al. 2015). The results of the current study suggest that this physiological trade-off could also be present in C. truncata, thus suggesting that this strategy could be utilised by multiple taxa to maintain homoeostasis when facing high pCO2 conditions, at least in the short term. However, in the isopod species examined this mechanism remains less well defined when compared to that of the polychaete S. spallanzanii. Furthermore, even though a high pCO2 induced downregulation of carbonic anhydrase has also previously been shown in other low-calcifying marine invertebrate species, such as the crab Carcinus maenas (Fehsenfeld et al. 2011), it is unlikely that this represents a long-term adaptive strategy. Instead this physiological plastic response may have evolved to extend aerobic scope under short-term exposure to high pCO2 conditions. This could be particularly relevant for groups such as isopods. These have a fairly low degree of calcification and are known to be able to successfully regulate internal acid–base balance. However, we know little about any effect of long-term exposure to high pCO2 conditions on acid–base regulation or the metabolic costs of maintaining homoeostasis (c.f. Wood et al. 2008; Seibel et al. 2012) which could, at least for some species be problematic. The isopod cuticle contains magnesium calcite which is highly soluble. Long-term exposure to OA-associated changes to oceanic carbonate chemistry could result in surface sea water becoming undersaturated with respect to this mineral (Feely et al. 2004; Andersson et al. 2008; Neues and Epple 2008). Therefore, the fact that it is likely that any long-term downregulation in carbonic anhydrase could ultimately result in respiratory function and acid–base processes being compromised (Henry 1996) means that this strategy is unlikely to be sustainable in the long term. This may be most true as organisms also undertake energy demanding processes, such as growth and reproduction. In contrast, in the current study there is a significant increase in carbonic anhydrase for the sensitive D. torelliae after exposure to high pCO2 conditions. This is consistent with what has previously been recorded for other marine calcifiers such as the bivalves Crassostrea virginica and Mercenaria mercenaria, for which exposure to high pCO2 has been shown to cause an increase in carbonic anhydrase concentration to maintain CaCO3 deposition (Ivanina et al. 2013). Whilst the role of carbonic anhydrase in shell calcification is fundamental for calcifying species, in both calcifying and non-calcifying marine invertebrates, this increase in carbonic anhydrase concentration is needed to ultimately maintain cellular homoeostasis through facilitating gas exchange and CO2 excretion. When these CO2 sensitive isopod species are faced with high pCO2 conditions it appears they are utilising the up or down regulation of carbonic anhydrase, as a physiological trade-off to ultimately extend their metabolic capacity. This also suggests that despite being CO2 sensitive, there may be differing degrees of sensitivity between closely related taxa with a species-specific degree of metabolic resilience being potentially advantageous to enable them to cope with future OA conditions. Furthermore, the identification of these kinds of alternative physiological strategies used by marine invertebrates when they are exposed to high pCO2 conditions may have important implications for our understanding of the generality of the physiological mechanisms involved in species responses to OA (Melzner et al. 2009; Stillman and Paganini 2015).
Dynamene bifida responds with a different metabolic fingerprint to the exposure to high and low pCO2 conditions. This species is known to thrive inside the high pCO2 vented area (Ricevuto et al. 2012) leading to the classification of this species as CO2 tolerant. Dynamene bifida significantly increases ATP production when transplanted from high to low pCO2 conditions, this being an approximately 20 % increase compared to individuals of D. bifida collected from and maintained inside the vents. This is a similar situation to that seen in the CO2 tolerant annelid species Platynereis massiliensis which is also found in large numbers inside the high pCO2 vent at Ischia (Lucey et al. 2015). When individuals of P. massiliensis are transplanted from high to low pCO2 conditions, this species also elevates its metabolic rate compared to that of individuals maintained inside the vented high pCO2 area (Calosi et al. 2013b). This suggests that this could be a multi-taxa response whereby the metabolism of vent individuals is maintained at high levels, compared to those that inhabit low pCO2 areas (Pörtner and Farrell 2008; Calosi et al. 2013b; Melzner et al. 2013). Furthermore, the fact that this increase in ATP production in D. bifida when transported to low pCO2 conditions is also not accompanied by a significant difference in l-lactate production could suggest that this species has the capacity to upregulate its metabolism without incurring the upregulation of anaerobic pathways. Overall, this could suggest that these species have metabolically adapted to high pCO2 conditions at the Ischia vent site.
The tolerant species, D. bifida is found throughout the Mediterranean (Torelli 1930; Ledoyer 1962; Holdich 1970; van der Land 2001; Kirkim et al. 2006; Koukouras 2010) in low CO2 conditions. Both a change in food quality (Garrard et al. 2014) and the nature of plant infochemicals (Zupo et al. 2015) have been cited as habitat attributes that can influence the distribution of isopods under ocean acidification conditions. However, it is possible that for D. bifida there are also ecological advantages, such as protection from predation and/or increased availability of food (Kroeker et al. 2011) for invading the high pCO2 vented area at Ischia.
The results of our study suggest that the distribution of the sphaeromatid isopod assemblage around the high pCO2 vent at Ischia may be explained, at least in part, by their ability to acclimatise or adapt to the high pCO2 conditions. However, other factors may also influence the distribution of these species. The life cycles of Cymodoce and Dynamene and the behaviour of juveniles are characterised on a diel basis, by habitat shifts which probably rely on a complex of environmental signals (e.g. Sanchez-Jerez et al. 1999). Previously, CO2 concentration has been shown to influence sphaeromatid behaviour (Alenius and Munguia 2012), including diel vertical migrations in vegetated biotopes (Dumay 1971) suggesting there may be, as yet further complex interactions of physiology and behaviour that may also influence the distribution of individual species around the high pCO2 vents at Ischia. Nonetheless, the ability of organisms to adjust their physiology to maintain metabolic homoeostasis via either adaptation or acclimatisation in response to pCO2 will influence the distribution of species around areas of high pCO2 conditions. Ultimately this can have genetic, ecological and conservation implications, which are particularly important when predictions are made about how marine life will respond to future global change scenarios, e.g. OA. Our results highlight how the importance of understanding the physiological responses of marine species to OA can assist us in making predictions about how marine communities may respond to this future environmental challenge. Physiological information can be used to promote active conservation exercises (Wikelski and Cooke 2006) and help identify priorities for the preservation of marine biodiversity and ecosystems function (Seijo et al. 2016).
References
Alenius B, Munguia P (2012) Effects of pH variability on the intertidal isopod, Paradella dianae. Mar Freshw Behav Physiol 45:245–259
Andersson AJ, Mackenzie FT, Bates NR (2008) Life on the margin: implications of ocean acidification on Mg-calcite, high latitude and cold-water marine calcifiers. Mar Ecol Prog Ser 373:265–273
Arnberg M, Calosi P, Spicer JI, Tandberg AHS, Nilsen M, Westerlund S, Bechmann RK (2013) Elevated temperature elicits greater effects than decreased pH on the development, feeding and metabolism of northern shrimp (Pandalus borealis) larvae. Mar Biol 160:2037–2048
Bennett AF (1987) Chapter 7—interindividual variability: an underutilized resource. In: Feder ME, Bennett AF, Burggren WW, Huey RB (eds) New directions in ecological physiology. Cambridge University Press, New York, pp 147–169
Branch TA, De Joseph BM, Ray LJ, Wagner CA (2012) Impacts of ocean acidification on marine seafood. Trends Ecol Evol 28:178–186
Byrne M (2011) Impact of ocean warming and ocean acidification on marine invertebrate life history stages: vulnerabilities and potential persistence in a changing ocean. Oceanogr Mar Biol 49:1–42
Caldeira K, Wickett ME (2003) Anthropogenic carbon and ocean pH. Nature 425:365
Calosi P, Rastrick SPS, Graziano M, Thomas SC, Baggini C, Carter HA, Hall-Spencer JM, Milazzo M, Spicer JI (2013a) Distribution of sea urchins living near shallow water CO2 vents is dependent upon species acid-base and ion-regulatory abilities. Mar Pollut Bull 73:470–484
Calosi P, Rastrick SPS, Lombardi C, de Guzman HJ, Davidson L, Jahnke M, Giangrande A, Hardege JD, Schulze A, Spicer JI, Gambi MC (2013b) Adaptation and acclimatization to ocean acidification in marine ectotherms: an in situ transplant experiment with polychaetes at a shallow CO2 vent system. Philos T Roy Soc B 368:20120448
Calosi P, Turner LM, Hawkins M, Bertolini C, Nightingale G, Truebano M, Spicer JI (2013c) Multiple physiological responses to multiple environmental challenges: an individual approach. Integr Comp Biol 53:660–670
Carter HA, Ceballos-Osuna L, Miller NA, Stillman JH (2013) Impact of ocean acidification on metabolism and energetics during early life stages of the intertidal porcelain crab Petrolisthes cinctipes. J Exp Biol 216:1412–1422
Ceballos-Osuna L, Carter HA, Miller NA, Stillman JH (2013) Effects of ocean acidification on early life-history stages of the intertidal porcelain crab Petrolisthes cinctipes. J Exp Biol 216:1405–1411
Cigliano M, Gambi MC, Rodolfo-Metalpa R, Patti FP, Hall-Spencer JM (2010) Effects of ocean acidification on invertebrate settlement at volcanic CO2 vents. Mar Biol 157:2489–2502
De Wit P, Dupont S, Thor P (2015) Selection on oxidative phosphorylation and ribosomal structure as a multigenerational response to ocean acidification in the common copepod Pseudocalanus acuspes. Evol Appl. doi:10.1111/eva.12335
Dumay D (1971) Écologie et biologie du genre Cymodoce (Isopoda: Flabellifera) dans la région de Marseille. Tethys 2:827–858
Edmunds PJ, Cumbo VR, Fan TY (2013) Metabolic costs of larval settlement and metamorphosis in the coral Seriatopora caliendrum under ambient and elevated pCO(2). J Exp Mar Biol Ecol 443:33–38
Egilsdottir H, Spicer JI, Rundle SD (2009) The effect of CO2 acidified sea water and reduced salinity on aspects of the embryonic development of the amphipod Echinogammarus marinus (Leach). Mar Pollut Bull 58:1187–1191
Fabry VJ, Seibel BA, Feely RA, Orr JC (2008) Impcts of ocean acidification on marine fauna and ecosystem processes. ICES J Mar Sci 65:414–432
Feely RA, Sabine CL, Lee K, Berelson W, Kleypas J, Fabry VJ, Millero FJ (2004) Impact of anthropogenic CO2 on the CaCO3 system in the oceans. Science 305:362–366
Fehsenfeld S, Kiko R, Appelhans Y, Towle DW, Zimmer M, Melzner F (2011) Effects of elevated seawater pCO2 on gene expression patterns in the gills of the green crab Carcinus maenas. BMC Genomics 12:488
Gambi MC, Musco L, Giangrande A, Badalamenti F, Micheli F, Kroeker KJ (2016) Distribution and functional traits of polychaetes in a CO2 vent system: winners and losers among closely related species. Mar Ecol Prog Ser 550:121–134
Garrard S, Gambi M, Scipione M, Patti F, Lorenti M, Zupo V, Paterson D, Buia M (2014) Indirect effects may buffer negative responses of seagrass invertebrate communities to ocean acidification. J Exp Mar Biol Ecol 461:31–38
Gunderson AR, Stillman JH (2015) Plasticity in thermal tolerance has limited potential to buffer ectotherms from global warming. Proc R Soc B Biol Sci 282:8
Hadfield MG (2000) Why and how marine-invertebrate larvae metamorphose so fast. Semin Cell Dev Biol 11:437–443
Hagerman L, Vismann B (1997) Oxygen binding characteristics of haemocyanin in the Baltic isopod Saduria entomon. Ophelia 46:141–151
Hall-Spencer JM, Rodolfo-Metalpa R, Martin S, Ransome E, Fine M, Turner SM, Rowley SJ, Tedesco D, Buia M-C (2008) Volcanic carbon dioxide vents show ecosystem effects of ocean acidification. Nature 454:96–99
Hauton C, Tyrrell T, Williams J (2009) The subtle effects of sea water acidification on the amphipod Gammarus locusta. Biogeosci Discuss 6:919–946
Henry RP (1996) Multiple roles of carbonic anhydrase in cellular transport and metabolism. Annu Rev Physiol 58:523–538
Hervant F, Mathieu J, Messana G (1997) Locomotory, ventilatory and metabolic responses of the subterranean Stenasellus virei (Crustacea, Isopoda) to severe hypoxia and subsequent recovery. C R Acad Sci Ser III 320:139–148
Holdich DM (1970) Distribution and habitat preferences of Afro-European species of Dynamene (Crustacea-Isopoda). J Nat Hist 4:419–438
IPCC (2014) Intergovernmental Panel on Climate Change 5th Assessment Report, New York
Ivanina AV, Dickinson GH, Matoo OB, Bagwe R, Dickinson A, Beniash E, Sokolova IM (2013) Interactive effects of elevated temperature and CO2 levels on energy metabolism and biomineralization of marine bivalves Crassostrea virginica and Mercenaria mercenaria. Comp Biochem Physiol A 166:101–111
Jakubowska M, Jerzak M, Normant M, Burska D, Drzazgowski J (2013) Effect of carbon dioxide-induced water acidification on the physiological processes of the Baltic isopod Saduria entomon. J Shellfish Res 32:825–834
Kirkim F, Kocatas A, Katagan T, Sezgin M (2006) Contribution to the knowledge of the free-living isopods of the Aegean Sea coast of Turkey. Turk J Zool 30:361–372
Klok C, Wijsman JWM, Kaag K, Foekema E (2014) Effects of CO2 enrichment on cockle shell growth interpreted with a dynamic energy budget model. J Sea Res 94:111–116
Knapp JL, Bridges CR, Krohn J, Hoffman LC, Auerswald L (2015) Acid-base balance and changes in haemolymph properties of the South African rock lobsters, Jasus lalandii, a palinurid decapod, during chronic hypercapnia. Biochem Bioph Res Co 461:475–480
Koukouras A (2010) Check-list of marine species from Greece. Aristotle University of Thessaloniki. Assembled in the framework of the EU FP7 PESI project
Kroeker KJ, Micheli F, Gambi MC, Martz TR (2011) Divergent ecosystem responses within a benthic marine community to ocean acidification. Proc Natl Acad Sci USA 108:14515–14520
Lannig G, Eilers S, Portner HO, Sokolova IM, Bock C (2010) Impact of ocean acidification on energy metabolism of oyster, Crassostrea gigas-Changes in metabolic pathways and thermal response. Mar Drugs 8:2318–2339
Ledoyer M (1962) Étude de la faune des herbiers superficiels de Zosteracées et de quelques biotopes d’algues littorales. Recl Trav St Mar End 25:117–235
Lewis CN, Brown KA, Edwards LA, Cooper G, Findlay HS (2013) Sensitivity to ocean acidification parallels natural pCO(2) gradients experienced by Arctic copepods under winter sea ice. Proc Natl Acad Sci USA 110:E4960–E4967
Lucey NM (2016) Improving our understanding of evolutionary persistence in an increasingly high CO2 world: Insight from marine polychaetes at a low pH vent system. PhD thesis, University of Pavia, Pavia, Italy and Plymouth University, Plymouth, UK
Lucey NM, Lombardi C, DeMarchi L, Schulze A, Gambi MC, Calosi P (2015) To brood or not to brood: are marine invertebrates that protect their offspring more resilient to ocean acidification? Sci Rep 5:12009
Lucey NM, Lombardi C, Florio M, DeMarchi L, Nannini M, Rundle S, Gambi MC, Calosi P (2016) An in situ assessment of local adaptation in a calcifying polychaete from a shallow CO2 vent system. Evol Appl. doi:10.1111/eva.12400
Maas AE, Wishner KF, Seibel BA (2012) The metabolic response of pteropods to acidification reflects natural CO2-exposure in oxygen minimum zones. Biogeosciences 9:747–757
Magozzi S, Calosi P (2015) Integrating metabolic performance, thermal tolerance, and plasticity enables for more accurate predictions on species vulnerability to acute and chronic effects of global warming. Glob Change Biol 21:181–194
Melatunan S, Calosi P, Rundle SD, Moody AJ, Widdicombe S (2011) Exposure to elevated temperature and pCO2 reduces respiration rate and energy status in the periwinkle Littorina littorea. Physiol Biochem Zool 84:583–594
Melatunan S, Calosi P, Rundle SD, Widdicombe S, Moody AJ (2013) Effects of ocean acidification and elevated temperature on shell plasticity and its energetic basis in an intertidal gastropod. Mar Ecol Prog Ser 472:155–168
Melzner F, Gutowska MA, Langenbuch M, Dupont S, Lucassen M, Thorndyke MC, Bleich M, Pörtner HO (2009) Physiological basis for high CO2 tolerance in marine ectothermic animals: pre-adaptation through lifestyle and ontogeny? Biogeosciences 6:2313–2331
Melzner F, Thomsen J, Koeve W, Oschlies A, Gutowska MA, Bange HW, Hansen HP, Koertzinger A (2013) Future ocean acidification will be amplified by hypoxia in coastal habitats. Mar Biol 160:1875–1888
Meyran J-C, Graf F, Fournié J (1987) Carbonic anhydrase activity in a calcium-mobilizing epithelium of the crustacean Orchestia cavimana during molting. Histochemistry 87:419–429
Munguia P, Alenius B (2013) The role of preconditioning in ocean acidification experiments: a test with the intertidal isopod Paradella dianae. Mar Freshw Behav Physiol 46:33–44
Neues F, Epple M (2008) Analysis of the composition of the cuticula (shell) of isopods. Netzsch Onset 4:6–8
Page LR (2009) Molluscan larvae: pelagic juveniles or slowly metamorphosing larvae? Biol Bull 216:216–225
Poore GCB, Bruce NL (2012) Global diversity of marine isopods (except asellota and crustacean symbionts). PLoS One 7:e43529
Pörtner HO (2008) Ecosystem effects of ocean acidification in times of ocean warming: a physiologist’s view. Mar Ecol Prog Ser 373:203–217
Pörtner HO, Farrell AP (2008) Ecology, physiology and climate change. Science 322:690–692
Rastrick SPS, Calosi P, Calder-Potts R, Foggo A, Nightingale G, Widdicombe S, Spicer JI (2014) Living in warmer, more acidic oceans retards physiological recovery from tidal emersion in the velvet swimming crab, Necora puber. J Exp Biol 217:2499–2508
Ricevuto E, Lorenti M, Patti FP, Scipione MB, Gambi MC (2012) Temporal trends of benthic invertebrate settlement along a gradient of ocean acidification at natural CO2 vents (Tyrrhenian sea). Biol Mar Medit 19:49–52
Ricevuto E, Vizzini S, Gambi MC (2015) Ocean acidification effects on stable isotope signatures and trophic interactions of polychaete consumers and organic matter sources at a CO2 shallow vent system. J Exp Mar Biol Ecol 468:105–117
Ries JB, Cohen AL, McCorkle DC (2009) Marine calcifiers exhibit mixed responses to CO2-induced ocean acidification. Geology 37:1131–1134
Rivest EB, Hofmann GE (2014) Responses of the metabolism of the larvae of Pocillopora damicornis to ocean acidification and warming. PLoS One 9:e96172
Rodolfo-Metalpa R, Houlbreque F, Tambutte E, Boisson F, Baggini C, Patti F, Jeffree R, Fine M, Foggo A, Gattuso J, Hall-Spencer JM (2011) Coral and mollusc resistance to ocean acidification adversely affected by warming. Nat Clim Change 1:308–312
Ross PM, Parker L, O’Connor WA, Bailey EA (2011) The impact of ocean acidification on reproduction, early development and settlement of marine organisms. Water 3:1005–1030
Saba GK, Schofield O, Torres JJ, Ombres EH, Steinberg DK (2012) Increased feeding and nutrient excretion of adult Antarctic krill, Euphausia superba, exposed to enhanced carbon dioxide (CO2). PLoS One 7:e52224
Sanchez-Jerez P, Barbera-Cebrian C, Ramos-Espla A (1999) Daily vertical migrations in the epifauna associated with Posidonia oceanica meadows. J Mar Biol Assoc UK 79:971–977
Seibel BA, Maas AE, Dierssen HM (2012) Energetic plasticity underlies a variable response to ocean acidification in the pteropod Limacina helicina antarctica. PLos One 7:e30464. doi:10.1371/journal.pone.0030464
Seijo JC, Villanueva-Poot R, Charles A (2016) Bioeconomics of ocean acidification effects on fisheries targeting calcifier species: a decision theory approach. Fish Res 176:1–14
Small DP (2013) The effects of elevated temperature and pCO2 on the developmental eco-physiology of the European lobster, Homarus gammarus (L.). PhD thesis, Plymouth University, Plymouth, UK
Small D, Calosi P, White D, Spicer JI, Widdicombe S (2010) Impact of medium-term exposure to CO2 enriched seawater on the physiological functions of the velvet swimming crab Necora puber. Aquat Biol 10:11–21
Small DP, Calosi P, Boothroyd D, Widdicombe S, Spicer JI (2015) Stage-specific changes in physiological and life-history responses to elevated temperature and pCO(2) during the larval development of the European lobster Homarus gammarus (L.). Physiol Biochem Zool 88:494–507
Somero GN (2005) Linking biogeography to physiology: evolutionary and acclimatory adjustments of thermal limits. Front Zool 2:1
Spicer JI, Raffo A, Widdicombe S (2007) Influence of CO2-related seawater acidification on extracellular acid-base balance in the velvet swimming crab Necora puber. Mar Biol 151:1117–1125
Stillman JH, Paganini AW (2015) Biochemical adaptation to ocean acidification. J Exp Biol 218:1946–1955
Torelli B (1930) Sferomidi del golfo di Napoli, revisione degli sferomoidi mediterranei. Pubbl Staz Zool 10:297–343
Turner LM, Ricevuto E, Massa Gallucci A, Gambi M-C, Calosi P (2015) Energy metabolism and cellular homeostasis trade-offs provide the basis for a new type of sensitivity to ocean acidification in a marine polychaete at a high-CO2 vent: adenylate and phosphagen energy pools vs. carbonic anhydrase. J Exp Biol 218:2148–2151
Urich K (1994) Comparative Animal Biochemistry. Springer, Berlin
Uthicke S, Liddy M, Nguyen HD, Byrne M (2014) Interactive effects of near-future temperature increase and ocean acidification on physiology and gonad development in adult Pacific sea urchin Echinometra sp. Coral Reefs 33:831–845
van der Land J (2001) Isopoda - excluding Epicaridea. In: Costello MJE, Emblow C, White RJ (eds) European register of marine species: a check-list of the marine species in Europe and a bibliography of guides to their identification: Collection Patrimoines Naturels 50. Muséum national d’Histoire naturelle, Paris, pp 315–321
Walther K (2010) Wirkungen von CO2 auf die Temperaturtoleranz und Fitnessindikatoren einer Crustaceenart aus verschiedenen Klimazonen. PhD thesis, University of Bremen, Bremen, Germany
Walther K, Anger K, Portner HO (2010) Effects of ocean acidification and warming on the larval development of the spider crab Hyas araneus from different latitudes (54 degrees vs. 79 degrees N). Mar Ecol Prog Ser 417:159–170
Walther K, Sartoris FJ, Portner H (2011) Impacts of temperature and acidification on larval calcium incorporation of the spider crab Hyas araneus from different latitudes (54 degrees vs. 79 degrees N). Mar Biol 158:2043–2053
Whiteley NM (2011) Physiological and ecological responses of crustaceans to ocean acidification. Mar Ecol Prog Ser 430:257–271
Widdicombe S, Spicer JI (2008) Predicting the impact of ocean acidification on benthic biodiversity: what can animal physiology tell us? J Exp Mar Biol Ecol 366:187–197
Wikelski M, Cooke SJ (2006) Conservation physiology. Trends Ecol Evolut 21:38–46
Wittmann AC, Pörtner H-O (2013) Sensitivities of extant animal taxa to ocean acidification. Nat Clim Change 3:995–1001
Wood HL, Spicer JI, Widdicombe S (2008) Ocean acidification may increase calcification rates, but at a cost. Proc R Soc B Biol Sci 275:1767–1773
Wood HL, Skold HN, Eriksson SP (2014) Health and population-dependent effects of ocean acidification on the marine isopod Idotea balthica. Mar Biol 161:2423–2431
Zheng CQ, Jeswin J, Shen KL, Lablche M, Wang KJ, Liu HP (2015) Detrimental effect of CO2-driven seawater acidification on a crustacean brine shrimp, Artemia sinica. Fish Shellfish Immunol 43:181–190
Zupo V, Maibam C, Buia M, Gambi M, Patti F, Scipione M, Lorenti M, Fink P (2015) Chemoreception of the seagrass Posidonia oceanica by benthic invertebrates is altered by seawater acidification. J Chem Ecol 41:766–779
Acknowledgments
We thank the staff of the Villa Dohrn—Benthic Ecology Centre (Ischia) for advice and technical support, particularly Capt. V. Rando for help with boat operations.
Funding
We acknowledge the support of the following funding: UKOARP NERC (NE/H017127/1) and Plymouth University to PC, ASSEMBLE (MetabolAdapt) to PC and LMT, Stazione Zoologica PhD fellowship to E. R., and the PON-MODO Project (Campania Region) to AMG. PC is in reception of a NSERC Discovery Grant.
Author contributions
PC, LMT and MCG conceived the study. ML classified the isopod species. All authors carried out the fieldwork. LMT was responsible for the biochemical determinations. LMT and PC carried out the statistical analyses. LMT and PC wrote the first draft of this manuscript with input from the other authors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Responsible Editor: A. E. Todgham.
Reviewed by A. L. Kelley and an undisclosed expert.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Turner, L.M., Ricevuto, E., Massa Gallucci, A. et al. Metabolic responses to high pCO2 conditions at a CO2 vent site in juveniles of a marine isopod species assemblage. Mar Biol 163, 211 (2016). https://doi.org/10.1007/s00227-016-2984-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-016-2984-x