Abstract
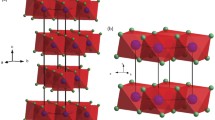
The thermal evolution of 10-Å phase Mg3Si4O10(OH)2·H2O, a phyllosilicate which may have an important role in the storage/release of water in subducting slabs, was studied by X-ray single-crystal diffraction in the temperature range 116–293 K. The lattice parameters were measured at several intervals both on cooling and heating. The structural model was refined with intensity data collected at 116 K and compared to the model refined at room temperature. As expected for a layer silicate on cooling in this temperature range, the a and b lattice parameters undergo a small linear decrease, α a = 1.7(4) 10−6 K−1 and α b = 1.9(4) 10−6 K−1, where α is the linear thermal expansion coefficient. The greater variation is along the c axis and can be modeled with the second order polynomial c T = c 293(1 + 6.7(4)10−5 K−1ΔT + 9.5(2.5)10−8 K−2(ΔT)2) where ΔT = T − 293 K; the monoclinic angle β slightly increased. The cell volume thermal expansion can be modeled with the polynomial V T = V 293 (1 + 8.0 10−5 K−1 ΔT + 1.4 10−7 K−2 (ΔT)2) where ΔT = T − 293 is in K and V in Å3. These variations were similar to those expected for a pressure increase, indicating that T and P effects are approximately inverse. The least-squares refinement with intensity data measured at 116 K shows that the volume of the SiO4 tetrahedra does not change significantly, whereas the volume of the Mg octahedra slightly decreases. To adjust for the increased misfit between the tetrahedral and octahedral sheets, the tetrahedral rotation angle α changes from 0.58° to 1.38°, increasing the ditrigonalization of the silicate sheet. This deformation has implications on the H-bonds between the water molecule and the basal oxygen atoms. Furthermore, the highly anisotropic thermal ellipsoid of the H2O oxygen indicates positional disorder, similar to the disorder observed at room temperature. The low-temperature results support the hypothesis that the disorder is static. It can be modeled with a splitting of the interlayer oxygen site with a statistical distribution of the H2O molecules into two positions, 0.6 Å apart. The resulting shortest Obas–OW distances are 2.97 Å, with a significant shortening with respect to the value at room temperature. The low-temperature behavior of the H-bond system is consistent with that hypothesized at high pressure on the basis of the Raman spectra evolution with P.



Similar content being viewed by others
References
Bauer JF, Sclar CB (1981) The “10-Å phase” in the system MgO-SiO2-H2O. Am Mineral 66:576–585
Comodi P, Fumagalli P, Montagnoli M, Zanazzi PF (2004) A single-crystal study on the pressure behavior of phlogopite and petrological implications. Am Mineral 89(4):647–653
Comodi P, Fumagalli P, Nazzareni S, Zanazzi PF (2005) The 10-Å phase: Crystal structure from X-ray single-crystal data. Am Mineral 90:1012–1016
Comodi P, Cera F, Dubrovinsky L, Nazzareni S (2006) The high pressure behaviour of the 10-Å phase: a spectroscopic and diffractometric study up to 42 GPa. Earth Planet Sci Lett 246:444–457
Fumagalli P, Stixrude L, Poli S., Snyder D (2001) The 10-Å phase: a high-temperature expandable sheet silicate stable during the subduction of hydrated lithosphere. Earth Planet Sci Lett 186:125–141
Güven N. (1971) The crystal structure of 2M 1 phengite and 2M 1 muscovite. Zeitschrift fur Kristallography 134:195–212
Horiuchi H, Morimoto N, Yamamoto K, Akimoto SI (1979) Crystal structure of Mg2SiO4 • 3Mg (OH)2, a new high-pressure structure type. Am Mineral 64:593–598
Kanzani M (1991) Stability of hydrous magnesium silicates in the mantle transition zone. Phys Earth Planet Inter 66:307–319
Ibers JA, Hamilton WC (eds) (1974) International tables for X-ray crystallography, vol. 4 Kinoch, Birmingham UK, pp 99–101
Miller AK, Guggenheim S, Koster van Groos A (1991) The incorporation of water in a high-pressure 2:1 layer silicate: A high pressure differential thermal analysis of the 10-Å phase. Am Mineral 76:106–112
Pawley AR, Redfern SAT, Wood BJ (1995) Thermal expansivities and compressibilities of hydrous phases in the system MgO-SiO2-H2O: talc, phase A and 10-Å phase. Contrib Mineral Petrol 122:301–307
Pawley AR, Wood BJ (1995) The high-pressure stability of talc and 10-Å phase: potential storage sites for H2O in subduction zones. Am Mineral 80:998–1003
Perrillat JP, Daniel I, Koga KT, Reynard B, Cardon H, Crichton WA (2005) Kinetics of antigorite dehydration: a real-time X-ray diffraction study. Earth Planet Sci Lett 236:899–913
Prewitt CT, Downs RT (1998) High-pressure crystal chemistry. In: Hemley RH (ed) Ultrahigh-pressure mineralogy, vol. 37 Reviews in mineralogy, mineralogical society of America, Wash. USA, pp. 293–317
Russell RL, Guggenheim S (1999) Crystal structures of near-end-member phlogopite at high temperatures and heat-treated Fe-rich phlogopite: the influence of O, OH, F site. Can Mineral 37:711–720
Sheldrick GM (1996) SADABS. Program for empirical absorption correction of area detector data. Institut für anorganische chemie, University of Göttingen, Germany
Sheldrick GM (1997) SHELX-97. Program for crystal structure determination. University of Göttingen, Germany
Spek AL (1997) HELENA. University of Utrecht, The Netherlands
Tutti F, Dubrovinsky LS, Nygren M (2000) High-temperature study and thermal expansion of phlogopite. Phys Chem Miner 27:599–603
Yamamoto K, Akimoto SI (1977) The system MgO-SiO2-H2O at high pressures and temperatures stability field hydroxyl-chondrodite, hydroxyl-clinohumite and 10-Å phase. Am J Sci 277:288–312
Weiss Z, Rieder M, Chmielovà M (1992) Deformation of coordination polyhedra and their sheets in phyllosilicates. Eur J Mineral 4:665–682
Wunder B, Schreyer W (1992) Metastability of the 10-Å phase in the system MgO-SiO2-H2O (MSH). What about hydrous MSH phases in subduction zones? J Petrol 33:877–889
Acknowledgments
Dr Andreas Schönleber is thanked for technical support with the temperature-dependent X-ray diffraction experiments. This research was supported by Italian MURST grants to P.F.Z. (COFIN 2005–2006, “Studio delle variazioni cristallochimiche indotte da temperatura e pressione nei minerali”) and to P.C. (COFIN 2004–2005, “Vincoli naturali (Ulten Zone, Italia) e sperimentali sul ruolo delle fasi idrate nei processi di interazione crosta-mantello”).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Zanazzi, P.F., Comodi, P., Nazzareni, S. et al. Behavior of 10-Å phase at low temperatures. Phys Chem Minerals 34, 23–29 (2007). https://doi.org/10.1007/s00269-006-0123-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00269-006-0123-9