Abstract
Purpose
This study investigated the effects of plants on the available pools of heavy metals and their re-supply potential in contaminated substrates in a short-term experiment using five metal-accumulating willow and poplar species/cultivars and in a longer-term experiment for Salix x reichardtii.
Material and methods
Five species of willow and poplar were grown in either soil or biosolids for short-term experiment (4 months). Further investigations of longer-term effects of plant on metal availability were conducted with S. x reichardtii grown in biosolids in a column (100 cm height and 37.5 cm diameter) experiment over a period of 12 months. Samples collected before and after experiments were determined for pH and bioavailability of metals using diffusive gradients in thin films (DGT). Various pools of metals in biosolids were determined by sequential extraction. Concentrations of heavy metals in plant material were determined.
Results and discussion
The concentration of metals determined by DGT (C DGT) and concentration of metals in pore water (C SOL) of Ni, Cu, Zn, and Cd in soil and biosolids generally decreased significantly compared to the initial measurements and were usually lower than those of the controls. However, C DGT and C SOL were higher in planted soil compared to those in the controls. There was a negative correlation between Ni, Zn, and Cd in plant shoots and C DGT in both soil and biosolids. The R values, the ratio of C DGT/C SOL calculated for Ni, Cd and Zn of planted substrates, were significantly higher than the corresponding R values of initial substrates. By contrast, R values for Cu showed little change. R values for Ni, Zn, and Cd were higher in planted biosolids compared to the unplanted biosolids. While S. x reichardtii leaf Cd, Ni, and Zn concentrations increased significantly over time, leaf Cu concentration declined. The patterns of plant uptake for the metals reflected the patterns observed by DGT and soil solution measurements of R. Sequential extraction of heavy metals from biosolids after 12 month’s experimentation confirmed that Cu was predominantly in the organic fraction.
Conclusions
The short-term effects of plants on the bioavailability of metals in soils and biosolids were different. The R values of cultivated treatments varied between species but were not significantly different from the control in most of the cases. The longer-term experiment indicated that both C DGT and C SOL of Ni, Zn, and Cd decreased significantly over time in both planted and unplanted treatments. The results of this study demonstrated that R values measured by DGT may be useful in assessing the potential bioavailability of heavy metals in soil and biosolids.
Similar content being viewed by others
1 Introduction
Phytoextraction is defined as the use of plants to remove contaminants from soils, sediments, waste material, or water by transfer into harvestable plant tissue (Kumar et al. 1995). In the last decade, hyperaccumulating plants have been tested to extract Ni, Zn, and Cd from soils (Baker et al. 1994; Robinson et al. 1998). Unfortunately, the use of hyperaccumulators in phytoextraction is often limited by low plant productivity and a narrow range of metals that are efficiently removed (Baker et al. 1991). As an alternative, fast-growing, high-biomass-producing, and metal-accumulating plants such as willows and poplars have shown considerable potential for phytoremediation of soils contaminated with heavy metals (Punshon et al. 1996; Huang et al. 1997). Willow (Salix viminalis) is believed to be sufficiently metal resistant to enable it to be used to decrease the load of plant-available heavy metals in contaminated soil (Dickinson 2000) and has been trialed for phytoextraction of Cd and Zn in contaminated soil in the field (Hammer et al. 2003).
Nutrients for plant growth are directly supplied from the soil solution. When plant roots deplete nutrient concentrations in the soil solution, supply is induced from the solid phase (Barber 1984). Heavy metals (e.g., Ni, Cu, Zn, and Cd) are taken up by plants during plant growth according to similar principles. As the heavy metals in the solution phase are continuously removed by the plant over the period of time, they will be continuously re-supplied from the solid phase. It has been suggested that free metal ions in soil solution are the dominant forms available for biota (Parker and Pedler 1997). The remaining portions of metal ions are bound with dissolved organic ligands or other complexes. Moreover, plants can alter the mobility and bioavailability of metals such as Cu in the rhizophere by modifying soil pH (Chaignon et al. 2002) or by releasing soluble organic compounds to complex Cu (Lombi et al. 2001; Chaignon and Hinsinger 2002). Tao et al. (2003) revealed a transformation from less bioavailable to more bioavailable fractions of Cu that may result from root-induced changes in dissolved organic carbon (DOC), redox potential, and microbial activity in the rhizosphere. Zhao et al. (2007) suggested that the influence of S. viminalis roots on metal solubility and Cu solubility was strongly related to DOC. They also indicated that S. viminalis water extraction directly influenced the amount of metal leaching. Understanding the bioavailability of metals and their behavior in plant and soil systems is the key to success in phytoremediation technology.
The technique of diffusive gradients in thin films (DGT) has been developed to mimic uptake of heavy metals by plant roots in soils (Zhang et al. 2001). It has shown good promise for measuring labile solution species and flux of available species of metals in soil (Zhang et al. 1998). The DGT–soil system mimics the dominant supply processes in soils that are diffusion and labile metal release (Davison et al. 2000). Pot trials have shown good correlations between plant metal uptake and metals measured in contaminated soils by DGT (Zhang et al. 2001; Davison et al. 2000; Zhang et al. 2004). Plant Zn concentration was highly correlated with Zn determined by DGT in soil, and it has therefore been suggested that DGT is a sensitive tool to assess Zn phytoavailability in contaminated agricultural soils (Cornu and Denaix 2006). DGT has been used to assess the potentially bioavailable metal directly in soils in the field (Nowack et al. 2004). Cattani et al. (2006) suggested that DGT can be used to predict the effects of cultivation on polluted soil.
Aged stockpiled biosolids and soils formerly used for land filtration of sewage at Melbourne Water’s Western Treatment Plant (WTP), Werribee, Victoria, Australia, are contaminated with multiple elements, particularly Cd, Cu, Ni, and Zn. Field trials to assess the suitability of willow and poplar for phytoextraction of heavy metals in soil and biosolids at WTP are currently being undertaken (Laidlaw et al. 2007). Biosolids are solid residues from wastewater treatment after either aerobic or anaerobic digestion processes and are substrates rich in organic matter. The chemical and physical properties of biosolids are different from those of soil. Thus, the effect of plants on metals in biosolids may be different to those in soil. Both contaminated soil and biosolids from WTP were used in this study.
A resin gel (Chelex, Bio-Rad) is recommended for use in the DGT unit, as it is selective for metal cations. The approximate order of cations selectivity shows that Zn2+ is more strongly bound than Cd2+ in nitrate or chloride solutions. The performance of the DGT to predict available Cd in the presence of high concentration of Zn has not been tested. Soil and biosolids used in this study have very high Zn concentrations in pore-water compared to Cd; the Zn concentration may affect the determination of Cd by DGT. Multiple metals contamination and the organic matter matrix of soils and biosolids (Table 1) from the WTP may also affect the ability of DGT to measure heavy metals in these materials. The standard laboratory test on the effects of high Zn and Ni concentrations on Cd determined by DGT was also conducted.
This study aimed to determine the effects of plants on the available pools of heavy metals (Ni, Cu, Zn, and Cd), and their re-supply potential in contaminated soil and biosolids in a short-term experiment (4 months) using five metal-accumulating willow and poplar species/cultivars and in a longer-term experiment (12 months) for S. x reichardtii grown in biosolids.
2 Materials and methods
2.1 Testing the effect of high Zn and Ni concentration on Cd determination by DGT
The effects of Ni and Zn at high concentrations on the ability of DGT to measure accurately the labile concentrations of Cd were tested using standard solutions (Zhang and Davison 1995). Three standard solutions containing three different mixtures of Cd, Ni, and Zn (1) 1, 5, and 10 mg L−1; (2) 1, 5, and 50 mg L−1; and (3) 1, 5, and 100 mg L−1, respectively, were used in the test. These solutions had the same ionic strength of 0.01-M NaNO3 and a pH of 5.8. Deployment times of 4 h were used. Details for testing DGT in standard solutions are fully described by Zhang and Davison (1995).
2.2 The reproducibility of DGT on soil and biosolids
Nine samples (four biosolids and five soils) collected from various phytoextraction experiments with a wide range of heavy metal concentrations (milligrams per kilogram): Cu (43–764), Ni (15–153), Zn (159–1502), and Cd (1–21) and pH (4.2–6.7) were used. In this trial, DGT was deployed for 20 h to avoid exceeding the capacity of the resin gel, as verified by calculations for the highest concentrations of metals in soils and biosolids. Samples were tested in triplicate. Details of DGT deployment for this test were similar to those for other samples in the study, as described in detail in the DGT deployment section. Intra-class correlation coefficients (ICC) were used to test the consistency or agreement of the DGT results using the SPSS statistical package.
2.3 Plant growth experiments
Two contaminated substrates (one soil and one biosolid) collected from site 65 West at the Western Treatment Plant (see Table 1) were used in a short-term experiment conducted over 4 months. Three species of willow (Salix alba, S. caprea, and S. x reichardtii) and two species of poplar (Populus deltoides and P. yunnanensis) selected from a previous study (Huynh et al. 2006) were used. Soil and biosolid samples were homogenized separately and passed through a 10-mm sieve. Selected healthy rooted cuttings were potted into 1-L pots with either soil or biosolids. The control treatments were soil or biosolids but without plants. Each treatment had three replicates, and each pot was watered daily with 300 mL of water.
Further investigations of longer-term effects (12 months) of plant presence on metal availability were conducted with only S. x reichardtii grown in biosolids. The samples used for this study were sampled from a column experiment (Huynh et al. 2008). The column had an approximately 70-cm layer of biosolids overlying a 20-cm layer of uncontaminated clay soil and a 5-cm pure sand layer at the bottom to prevent particulate leaching. Mature S. x reichardti cuttings previously grown in biosolids were transferred into the columns and grown for a further 12 months. Columns without plants were used as controls. Biosolid samples were collected at 0.25 m below the surface of all the columns; this corresponded to the depth with the greatest root density. Plants were watered with 3.6 L per day per plant.
2.4 Soil and biosolid sample analysis
Soil and biosolids sampled before and after 4 months of plant growth and the biosolids collected from the column experiment after 12 months plant growth were air-dried and sieved to <2 mm prior to analysis. The samples were shaken with distilled water at a ratio of 1:5 soil:water for 1 h and then the pH was determined using a calibrated Inlab 413 pH probe (Metter Toledo Inc., Columbus, OH, USA). The samples were incubated at 65% of maximum water-holding capacity (MWHC) for 4 days and then wetted to 85% MWHC for 24 h prior to DGT deployment. Details of the DGT procedure have been fully described in a previous study (Zhang et al. 1998). In this experiment, the deployment time was 20 h. The total dissolved metal (C SOL) of soil and biosolids was centrifuge extracted from the same soil and biosolids samples that were used to determine C DGT by DGT devices. Samples of the DGT eluate (C DGT) and the soil/biosolids solutions (C SOL) were analyzed by inductively coupled plasma–mass spectrometry (ICP-MS, X-Series, Thermo Fisher Scientific, Waltham, MA, USA)
A sequential extraction scheme was used to determine the various pools of metals in biosolids after a 12 month phytoextraction. Metals in the biosolids were extracted into 4 fractions: (F1) soluble/exchangeable (0.1 M MgCl2 pH 7, 2 successive extractions), (F2) organic matter-bound (5% NaOCl pH 8.5, reacted in water bath at 90–95°C, 5 successive extractions), (F3) Fe/Mn oxyhydroxides-bound (0.2 M oxalic acid + 0.2 M ammonium oxalate + 0.1 M ascorbic acid adjusted to pH 3 with NH4OH and treated in a water bath at 90–95°C, 5 successive extractions), and (F4) residual (1:3 HNO3:HCl acid mixture treated in a water bath at 95–100°C for at least 5 h) (Ahnstrom and Parker 1999). Plant materials sampled at 4 and 12 months’ experimental treatment were digested using concentrated HNO3 to determine concentrations of heavy metals in the dry matter. Digests and sequential extraction samples were all analyzed for metals by axial inductively coupled plasma–optical emission spectrometry (ICP-OES, Vista AX, Varian Australia Pty Ltd, Melbourne, Australia).
3 Results and discussion
3.1 Effect of Zn and Ni on the determination of Cd by DGT
The quotients of concentrations of Ni, Zn, and Cd determined by DGT (C DGT) and the concentrations measured directly in the immersion testing solution (C SOL) were not significantly different for any of the standard solutions. Testing DGT using standard solutions should yield results within 10% of the actual concentrations (Zhang and Davison 1995). The differences between C DGT and C SOL for Cd, Ni, and Zn in all the three mixed standard solutions were within 3%–8%. Clearly, high Zn concentrations (100 mg L−1) did not affect the Cd concentration determined by DGT within the deployment time that did not exceed the capacity of resin gel.
3.2 The reproducibility of DGT measurements using WTP soil and biosolids
ICC values for all the four metals were greater than 0.90. The results showed very good reproducibility (RSD of replicates within 10%) of the DGT measurements across a wide range of concentrations for Cu, Ni, Zn, and Cd in both soil and biosolids from the WTP.
3.3 Short-term effects of plants on the availability of heavy metals in soil and biosolids
In the soil group, Cd, Cu, Ni, and Zn measured by DGT (C DGT) and in pore water (C SOL) of the soil after 4 months plant growth were generally higher than in the absence of plants (Table 2). Compared to other species, Salix x reichardtii showed the highest C DGT for Cd, Ni, and Zn. The increase in C DGT and C SOL of metals in soils under cultivation can be attributed mostly to the effect of plant roots that consume or release O2 that may cause alternations in soil redox potential (Marschner 1995). Microbial activity can stimulate the release of organic carbon from roots and produce organic acids (Marschner 1995) that will decrease soil pH. In our experiment, the pH of soils with plants was generally 0.2 pH units lower than that found for the control soil and 0.4 pH units lower than the initial soil pH (Table 3). This decrease of pH in the control soil compared to the initial soil pH (see Table 3) was most likely due to leaching processes. A previous study on the short-term (75 days) effects of willow roots on metal extractability in dredged sediment indicated that root growth increased extractability of Cd, Zn, and Cu, and extractable metals correlated well with pH and soil moisture content (Vervaeke et al. 2004). This implies that a decrease in pH in soil was associated with the aeration caused by plant growth. Cattani et al. (2006) suggested that maize cultivation decreased soil pH and increased DOC in rhizosphere of vineyard soil and consequently increased both C SOL and C DGT of Cu of the rhizosphere.
Within the biosolids group, C DGT values of Cu, Ni, Zn, and Cd for most species were significantly lower than for the control biosolids. Salix x reichardtii (Cu, Ni, and Cd) and P. yunnanensis (Zn and Cd) were exceptions (see Table 2). The C SOL of cultivated biosolids also tended to be lower than the control biosolids, but values were not statistically significant for Ni, Zn, and Cd. The decrease in metal concentrations in cultivated biosolids appeared to be caused mainly by uptake by plants. Plant tissue metals increased as C DGT and C SOL metals decreased. The effect of roots on metal release from biosolids may not be detectable against the high background concentration of metals in the biosolids. In contrast to the findings for soils, the pH of cultivated biosolids was slightly higher than for the controls. Biosolids have high organic matter content, reactive Fe, and exchangeable Al (see Table 1) and thus have a greater pH buffering capacity. Organic-rich materials have greater reserve acidity than active acidity, resulting in a high pH buffering capacity. Hydrous Fe oxides are also known to be important in the adsorption of metal ions in soil (Alloway and Jackson 1991).
An alternative explanation is that the increase in pH may be mainly due to the decomposition of organic matter, which is influenced by a combination of factors, including excess water from irrigation, temperature conditions, and micro-organism activity. The decomposition of organic matter releases cations such as K+, Na+, Mg2+, and Ca2+ into the exchangeable phase. These cations displace protons and aluminium from exchange sites and thus increase the base saturation, which causes pH to increase (Bessho and Bell 1992). Hoyt and Turner (1975) suggested that release of NH3 from decomposition of organic nitrogen can also elevate pH. The decomposition and removal of organic anions (e.g., carboxyl groups) may also increase the pH (Barekzai and Mengel 1993).
Changes in pH of soils generally occur as a result of the differential uptake rates of cations and anions by plant roots (Nye 1981). Protons are released to compensate for excess positive charges, when a large net uptake of cations occurs. Hydroxyl or bicarbonate ions are released if plant roots take up an excess of anions (Haynes 1990). The lower soil pH and higher biosolids pH compared to those in the controls suggest that the effects of plant roots were different in soil and biosolids. The changes of pH were consistent with the availability of metals (C DGT and C SOL) in soil and biosolids (see Table 2). Changes in metals concentration (C DGT and C SOL) in the solution phase of soil and biosolids in the 4-month experiment is due to both plant uptake and roots, indirectly mobilizing metals from the solid phase into the solution phase, most likely by associated changes in pH.
Previous studies involving relatively short-term growth experiments in a range of soils indicated that C DGT of metals in soils was highly positively correlated to the concentration of Zn (Zhang et al. 2004; Nowack et al. 2004; Nolan et al. 2005), Pb, Cd (Nolan et al. 2005) and Cu (Zhang et al. 2001; Nolan et al. 2005) in plant tissue. In this study where DGT was deployed in soil and biosolids after 4 months of cultivation, plots of plant tissue metal concentration of five species versus C DGT of soil and biosolids separately showed negative correlations for Ni, Zn, and Cd in both soil and biosolids groups/treatments (Fig. 1), with correlation coefficients, R 2, ranging from 0.6–0.8. These results are consistent with the plants depleting Ni, Zn, and Cd from the solution phase of soil and biosolids. The lower C DGT and C SOL observed in soil and biosolid samples where the rate of metal uptake was high (with higher metal concentrations in plants) suggest that significant amounts of labile metals were removed by plants (see Table 2). For both soil and biosolids, Cu in plant tissue was not correlated with Cu (C DGT) implying that the processes for Cu are different.
The R values for soil and biosolids, before and after the 4-month experiment, were calculated to determine available pools of heavy metals and their re-supply potential during plant growth. The results (Fig. 2) showed that the R values for Ni, Cu, Zn, and Cd in both soil and biosolids that have been cultivated or simply leached (control) were generally higher than the corresponding R values of soil and biosolids before plant growth (except for S. alba grown in soil). They indicated that the fraction of available metals (C DGT) to the total dissolved metals in soil solution (C SOL) of soil and biosolids tended to increase during the experiment. However, R values in cultivated treatments varied between species and, in most cases, were indistinguishable from the control, suggesting that leaching was the dominate process.
DGT determinations combine a measure of soil solution metals and an estimate of the metals that can be rapidly supplied from the solid phase. The incorporation of the kinetics of metal supply from solid to solution phase in the DGT measurement is important for determining Cd and Zn (Nolan et al. 2005). The lower R values of Cd and Zn in soil and biosolids before phytoextraction indicate a slower re-supply rate of heavy metals from the solid phase to the solution phase. Similar values for control and cultivated soils suggest that the duration of this experiment (4 months) may not be long enough to detect the effect of plants on the re-supply parameters for the metals studied. This re-supply rate may be influenced by the high content of organic matter and Fe in both soil and biosolids (see Table 1). Organic ligands are known to reduce free metal activity in the soil solution of sludge-amended soil (O’Connor et al. 1984) and hydrous oxides of Fe are important adsorbents of metals in soils (Alloway and Jackson 1991). The results for S. x reichardtii appear to be anomalous, with C DGT (see Table 2 and Fig. 3) and R values (see Figs. 2 and 4) for Cd, Ni, and Zn being higher in cultivated soil than in soils for other species. This may be due to the effect of S. x reichardtii on the available pool of metals in the solution phase or an increase in the re-supply rate of heavy metals from the solid phase to the solution phase.
3.4 Longer-term effects of S. x reichardtii on the availability of heavy metals in biosolids
The concentration of metals (Ni, Cu, Zn, and Cd) determined by DGT (C DGT) and in pore-water (C SOL) of biosolids after 12 months cultivated with S. x reichardtii decreased dramatically compared to 4 months and before cultivation (see Fig. 3). The reduction of heavy metals in biosolids under cultivation is assumed to be due to plant uptake and leaching. In the control treatment, the loss is only from leaching. The difference between the control and the plant treatments is assumed to be due to plant uptake. However, re-supply of metals into the soil solution phase may be more rapid in the plant treatment compared to the control due to the effect of plant roots. The pH of control biosolids (4.9) was slightly higher than in biosolids with S. x reichardtii (4.7) after 12 months. The absence of plant roots and water uptake by plants may have maintained higher soil moisture content in the control column and thus the greater volume of leachate within the control columns compared to planted columns. However, average concentrations of metals in the leachate of control treatments were generally lower than treatments with plants (data not presented here). Consequently, mass balance of metals in the columns showed that the percentages of total Ni, Cu, Zn, and Cd (31%, 10%, 25%, and 18%, respectively) lost by leaching in planted columns were higher than that of the controls (29%, 4%, 22%, and 11%, respectively). The higher average concentration of metals in the leachate from planted columns could result from a higher concentration of metals in the solution phase associated with the plant roots. Except for Cu, the loss of metals (C DGT and C SOL) from the biosolids with plants is significantly higher than from the control biosolids (see Fig. 3.), consistent with S. x reichardtii extracting and mobilizing significant amounts of metals in the system during the 12 months’ trial.
The R value measured in biosolids where S. x reichardtii was grown for different times (see Fig. 4) showed that over the 12-month period, the available fraction of Cu remained steady and increased significantly for Ni, Zn, and Cd. R values for Ni, Zn, and Cd in S. x reichardtii treatments are slightly but significantly higher than the control treatment (p < 0.05) after 12 months. This suggests that S. x reichardtii actively modified the soil solution/solid phase balance. Although this is only a small effect, the greater proportion of metal in the solid phase may affect the bioavailability of Ni, Zn, and Cd, and indeed, there is some correspondence with the amount of metal accumulated in plant tissues over the phytoextraction period (Fig. 5).
Nickel, Zn, and Cd concentrations in S. x reichardtii leaves increased significantly after 12 months of phytoextraction, whereas Cu concentrations decreased (see Fig. 5). It appears that S. x reichardtii did not take up Cu in the later stages of the experiment. This could reflect the reduced availability of Cu in biosolids, possibly due to progressive strong binding of Cu to organic compounds (Garnett et al. 1987). In the solution phase of both sludge and sludge/soil mixtures, Cu can be present as complexes with relatively high molecular weight organic ligands (Alloway and Tills 1984). In general, a critical deficiency of Cu occurs in plants when Cu is in the range of 1–5 mg kg−1 dry weight (Marschner 1995). The Cu concentration in S. x reichardtii leaves after 12 month’s growth in biosolids was 6 mg kg−1 (DW), suggesting that S. x reichardtii may have been close to the deficiency threshold value. The minimal availability and re-supply of Cu in biosolids would result in a decrease in tissue Cu concentrations as uptake of Cu fails to keep pace with plant growth. The observed changes of Cu concentrations in S. x reichardtii are consistent with the measured R values for Cu not changing over a 12-month period. Although the total concentrations of Cu are higher than Ni in biosolids (see Table 1), there is much less uptake of Cu by either the plant (see Fig. 5) or DGT (see Fig. 3), so the reservoir of available Cu is more likely to be maintained. This deduced different distribution of Cu in the solid phase is supported by direct measurements (Fig. 6). Zinc and Cd were highest in the soluble/exchangeable and organic matter fractions. Nickel was found in equal amounts in three fractions (soluble/exchangeable; organic matter bound; Fe/Mn oxides bound). However, Cu was very low in the soluble/exchangeable fraction and mainly bound to the organic matter (see Fig. 6).
Concentrations of Ni, Zn, and Cd in the soluble/exchangeable and organic metals fractions of biosolids where plants had been grown were significantly lower than in the control biosolids (p < 0.05). For Ni and Zn, the Fe–Mn oxide metals fractions of biosolids with plant treatments were also lower than the controls. The organic matter-bound Cu in cultivated biosolids was significantly lower than the control biosolids (p < 0.01). The latter indicates that the Cu supplied to the solution phase for plant uptake was mainly from the organic matter fraction. This finding supports the previous results, which indicated that S. x reichardtii increased the re-supply of metals from the solid to the solution phase.
4 Conclusions
The short-term effects of plants on the bioavailability of metals in soils and biosolids were different, with the different pH and total metal concentrations of the substrates most probably being major influences. The R values (C DGT/C SOL) of watered soils and biosolids after 4 months tended to increase due to leaching, lowering C SOL. The R values of cultivated treatments varied between species but were not significant different from the control in most of the cases. The higher C DGT and R values of Cd and Zn in soils cultivated with S. x reichardtii compared to other species indicate that this plant species may affect the available metal pools.
The longer-term experiment showed that both C DGT and C SOL of Ni, Zn, and Cd decreased significantly overtime in both planted and control biosolid treatments. However, the R value quotients of available metals (C DGT) to total dissolved metals (C SOL) of planted biosolids were higher than that of the control biosolids, reflecting the influence of S. x reichardtii on the availability of Ni, Zn, and Cd in biosolids. Salix x reichardtii did not take up Cu effectively in the later stages of the experiment, and its effect on the availability of Cu in biosolids was not detected. These findings were supported by the analysis of metals fractions by sequential extractions, which indicated that the re-supply of Ni, Zn, and Cd in the solid phase mainly came from dissolved and exchangeable fractions, whereas Cu was released from the organic matter fractions. The controls on Cu would have been clearer had DOC been measured. The results of this study demonstrated that R values measured by DGT may be useful in assessing the potential bioavailability of heavy metals in soil and biosolids.
References
Ahnstrom ZS, Parker DR (1999) Development and assessment of a sequential extraction procedure for the fractionation of soil cadmium. Soil Sci Soc Am J 63:1650–1658
Alloway BJ, Tills AR (1984) Copper deficiency in world crops. Outlook Agric 13:32–42
Alloway BJ, Jackson AP (1991) The behavior of heavy metals in sewage sludge-amended soils. Sci Total Environ 100:151–176
Baker AJM, Reeves RD, McGrath SP (1991) In situ decontamination of heavy metal polluted soil using crops of metal-accumulating plants—a feasibility study. In: Hinchee RE, Olfenbuttel RF (eds) In situ Bioreclamation. Butterworth-Heinemann, Boston, pp 600–605
Baker AJM, McGrath SP, Sidoli CMD, Reeves RD (1994) The possibility of in situ heavy metal decontamination of pooled soil using crops of metal-accumulating plants. Resour Conserv and Recycl 11:41–49
Barber SA (1984) Nutrient uptake by plant roots growing in soil. In: Soil Nutrient Bioavailability: A Mechanistic Approach. John Wiley & Sons Inc., New York, pp 90–113
Barekzai A, Mengel K (1993) Effect of microbial decomposition of mature leaves on soil pH. Z Pflamzenerahr Bodenk 156:93–94
Bessho T, Bell LC (1992) Soil solid and solution phase changes and mung bean response during amelioration of aluminium toxicity with organic matter. Plant Soil 140:183–196
Cattani I, Fragoulis G, Boccelli R, Capri E (2006) Copper bioavailability in the rhizophere of maize (Zea mays L.) grown in two Italian soils. Chemosphere 64:1972–1979
Chaignon V, Hinsinger P (2002) Fe-deficiency increases Cu acquisition by wheat cropped in Cu-contaminated vineyard soil. New Phytol 154:121–130
Chaignon V, Bedin F, Hinsinger P (2002) Copper bioavailability and rhizhosphere pH changes as effected by nitrogen supply for tomato and oilseed rape cropped on an acidic and a calcareous soil. Plant Soil 243:219–228
Cornu JY, Denaix L (2006) Prediction of zinc and cadmium phytoavailability within a contaminated agricultural site using DGT. Environ Chem 3:61–64
Davison W, Hooda P, Zhang H, Edwards AC (2000) DGT measured fluxes as surrogates for uptake of metals by plants. Adv Environ Res 3:550–555
Dickinson NM (2000) Strategies for sustainable woodland on contaminated soils. Chemosphere 41:259–263
Garnett K, Kirk PWW, Lester JN, Perry R (1987) Assessment of the interactions of metals and nitrilotriacetic acid in soil/sludge mixtures. Water Air Soil Poll 34:55–69
Hammer D, Kayser A, Keller C (2003) Phytoextraction of Cd and Zn with Salix viminalis in field trials. Soil Use Manage 19:187–192
Haynes RJ (1990) Active ion uptake and maintenance of cation-anion balance: a critical examination of their role in regulating rhizosphere pH. Plant Soil 126:247–264
Hoyt PB, Turner RC (1975) Effects of organic materials added to very acid soils on pH, aluminum, exchangeable NH4, and crop yields. Soil Sci 119:227–237
Huang JW, Chen J, Cunningham SD (1997) Phytoextraction of lead from contaminated soils. In Kruger TAAEL, Coats JR (ed). Phytoremediation of Soils and Water Contaminants, American Chemical Society, pp 283–298
Huynh TT, Baker AJM, Laidlaw WS, Singh B, Gregory D (2006) Survival, tolerance and heavy metal transfer factors for willow and poplar cultivars grown in contaminated soil and aged biosolids. In Proceedings of the Conference, Biosolids Speciality III, Australian Water Association, Australia
Huynh TT, Laidlaw WS, Singh B, Gregory D, Baker AJM (2008) Effects of phytoextraction on heavy metal concentrations and pH of pore-water of biosolids determined using an in situ sampling technique. Environ Pollut 156:874–882
Kumar PBAN, Dushenkov V, Motto H, Raskin I (1995) Phytoextraction—the use of plants to remove heavy metals from soils. Environ Sci Technol 29:1232–1238
Laidlaw WS, Gregory D, Huynh TT, Godino M, Baker AJM (2007) Phytoextraction of trace elements from an aged biosolids stockpile by willow (Salix) after one year. In: Zhu et al (eds) Biogeochemistry of trace elements: environmental protection, Remediation and Human Health. Tsinghua University Press, Beijing, pp 190–191
Lombi E, Wenzel W, Gobran GR, Adriano DC (2001) Dependency of phytoavailability of metals on indigenous and induced rhizosphere process: a review. In: Gobran GR et al (eds) Trace elements in the Rhizosphere. CRC Press, London, New York, Washington, DC, pp 4–23
Marschner H (1995) Functions of mineral nutrients: micronutrients. In: Mineral Nutrition of Higher Plants, Academic Press, pp 313–404
Nolan LA, Zhang H, McLaughlin JM (2005) Prediction of zinc, cadmium, lead, and copper availability to wheat in contaminated soils using chemical speciation, diffusive gradients in thin films, extraction, and isotopic dilution techniques. J Environ Qual 34:496–507
Nowack B, Koehler S, Schulin R (2004) Use of diffusive gradient in thin-film (DGT) in undisturbed field soils. Environ Sci Technol 38:1133–1138
Nye PH (1981) Changes of pH across the rhizosphere induced by roots. Plant Soil 61:7–26
O’Connor GA, Essington ME, Elrashidi M, Cline G (1984) Trace metal sorption in sludge amended soils. In Environmental Contamination (UNEP), CEP Consultants, Edinburgh, pp 225–231
Parker DR, Pedler JF (1997) Reevaluating the free-ion activity model of trace metal availability to higher plants. Plant Soil 196:223–228
Punshon T, Dickinson NM, Lepp NW (1996) The potential of Salix clones for bioremediating metal polluted soil. In: Heavy Metals and Trees. Institute of Chartered Foresters, Edinburgh, pp 93–104
Robinson BH, Leblanc M, Petit D, Brooks RR, Kirkman JH, Gregg PEH (1998) The potential of Thlaspi caerulescens for phytoremediation of contaminated soils. Plant Soil 203:47–56
Tao S, Chen FL, Xu J, Cao J, Li BG (2003) Changes of copper speciation in maize rhizosphere soil. Environ Pollut 122:447–454
Vervaeke P, Tack FMG, Lust N, Verloo M (2004) Short- and long-term effects of the willow root system on metal extractability in contaminated dredged sediment. J Environ Qual 33:976–983
Zhang H, Davison W (1995) Performance characteristics of diffusion gradients in thin film for the in situ measurement of trace metals in aqueous solution. Anal Chem 67:3391–3400
Zhang H, Davison W, Knight B, McGrath S (1998) In situ measurements of solution concentrations and fluxes of trace metals in soils using DGT. Environ Sci Technol 32:704–710
Zhang H, Zhao FJ, Sun B, Davison W, McGrath S (2001) A new method to measure effective soil solution concentration predicts copper availability to plants. Environ Sci Technol 35:2602–2607
Zhang H, Lombi E, Smolders E, McGrath S (2004) Kinetics of Zn release in soils and prediction of Zn concentration in plants using diffusive gradients in thin-films (DGT). Environ Sci Technol 38:3608–3613
Zhao L, Schulin R, Nowack B (2007) The effects of plants on the mobilization of Cu and Zn in soil columns. Environ Sci Technol 41:2770–2775
Acknowledgments
The authors would like to thank the University of Melbourne for a Postgraduate Overseas Research Experience Scholarship (PORES), Melbourne Water Corporation, and NoMiracle (a program funded by the European Commission under contract 003956) for financial assistance to allow T.T. Huynh to conduct the DGT work at Lancaster University, UK.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Jean-Paul Schwitzguébel
Rights and permissions
About this article
Cite this article
Huynh, T.T., Zhang, H., Laidlaw, W.S. et al. Plant-induced changes in the bioavailability of heavy metals in soil and biosolids assessed by DGT measurements. J Soils Sediments 10, 1131–1141 (2010). https://doi.org/10.1007/s11368-010-0228-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-010-0228-0


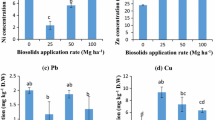






 and 12 months
and 12 months  growth in biosolids. The asterisks indicate a significant differences between two means at *p < 0.05 and **p < 0.01, respectively (t test)
growth in biosolids. The asterisks indicate a significant differences between two means at *p < 0.05 and **p < 0.01, respectively (t test)
 and the control (no plant)
and the control (no plant)  . The asterisks indicate a significant differences between two means at *p < 0.05 and **p < 0.01, respectively (t test)
. The asterisks indicate a significant differences between two means at *p < 0.05 and **p < 0.01, respectively (t test)