Abstract
Gas-condensate reservoirs experience significant productivity losses as reservoir pressure drops below the dew point due to condensate accumulation and water blocking and the subsequent reduction in gas relative permeability. One potential way to overcome this problem is to alter reservoir wettability to gas-wetting to reduce condensate accumulation and water blocking in the near wellbore and maintain high productivity. The major goal of this work was to study the mobility of the gas and liquid phase (both water and oil) before and after wettability alteration from strong liquid-wetting to intermediate gas-wetting. For this purpose, in addition to relative permeability measurements, we also conducted various other tests to demonstrate that liquid mobility can be improved significantly due to wettability alteration. As wettability modifiers, fluorinated polymers are capable of delivering a good level of oil and water repellency to the rock surface, making it intermediate gas-wet and alleviating such liquid blockage under high temperature. The contact angle analyses, capillary rise, flow tests, and imbibition spontaneous tests were used to estimate the effect of treatments on wettability. Experimental results demonstrated that the fluorinated polymers can alter the wettability of cores from strong liquid-wetting to gas-wetting, which could decrease the amount of water invaded and resided in the gas formation. Core flood test results demonstrated that the relative permeabilities of both the gas and the liquid phases were increased significantly after the wettability alteration to preferential gas-wetness. The residual liquid saturation was decreased, and the gas production was enhanced greatly due to the wettability alteration. These results imply that gas well deliverability may increase substantially when wettability is altered to intermediate gas-wetting. Efficiency in the extraction of natural gas is important to improve the productivity of gas-condensate reservoirs where liquid accumulates, which is beneficial for the economic and environmental considerations of the oil and gas industry.
Similar content being viewed by others
Introduction
A sharp reduction in gas well deliverability is often observed in many low-permeability gas-condensate reservoirs even at very high reservoir pressure. The decrease in well deliverability is attributed to condensate accumulation and water blocking (Li and Firoozabadi 2000; Allen and Roe. 1950; Barnum. 1995; Wagner and Leach 1958). As the pressure drops below the dew point, liquid accumulates at the wellbore in high saturations reducing gas relative permeability (Tang and Firoozabadi 2003; Fahes and Firoozabadi 2005), the result is a decrease in the gas production rate. Gas deliverability often increases after removal of water and condensate phases from the near-wellbore region. Several techniques have been used to increase gas well deliverability after the initial decline. Hydraulic fracturing and solvent injection are implemented in order to remove the accumulated liquid (Tannich 1975; Holditch 1979). The increased rates were, however, only sustained for a period. The approach is not a permanent solution to the problem as the condensate bank will form again. On the other hand, when hydraulic fracturing is used by injecting aqueous fluids, the clean-up of water accumulation from the formation after fracturing is essential to obtain an increased productivity. Water is removed in two phases, immiscible displacement by gas, followed by vaporization by the expanding gas flow (Mahadevan and Sharma 2003). Due to the low permeability and the wettability characteristics, it takes a long time to perform the clean-up; in some cases, as little as 10–15 % of the water load could be recovered. In a new approach, Li and Firoozabadi examined the alteration of wettability to intermediate gas-wetting by chemical treatment of the rock. A major factor in liquid accumulation is the liquid’s low mobility because of strong liquid-wetting (Walker et al. 2005; Zuluaga and Monsalve 2003). By altering the wettability of the rock from liquid-wetting to intermediate gas-wetting, an increase in liquid mobility is achieved, preventing the accumulation of liquid in high saturations and resulting in high rates of gas production (Boom and Zeelenberg 1996; Fahes and Firoozabadi 2005). The work of Firoozabadi and coworkers on wettability alteration may have a practical application in improving gas effective permeability due to the prevention of liquid accumulation in high saturations at the wellbore; the main requirement is the permanent alteration of wettability. Tang and Firoozabadi performed wettability alteration and measured the effect of wettability alteration on increased liquid mobility. The major goal of this work was to study the mobility of the gas and liquid phase (both water and oil) before and after wettability alteration from strong liquid-wetting to intermediate gas-wetting. For this purpose, in addition to relative permeability measurements, we also conducted various other tests to demonstrate that liquid mobility can be improved significantly due to wettability alteration. The alteration of wettability was achieved by treating the rock with the Zonyl8740 solution at 24 °C. This paper is organized along the following lines: First, the description of rocks, model reservoir fluids, chemicals used in treating the rocks, and the treatment procedure were presented. Then, the measurements of contact angle, imbibition, and flow testing were described, and in the section that follows, the results of these measurements in treated and untreated cores are presented. At the end, conclusions from the work were drawn. Note that the wettability alteration to gas-wetness may also be applied in drilling and completion to reduce the water loss to tight gas formation, in water shutoff in tight gas reservoirs with bottom water, and acid-fracturing to contain secondary formation damage in low-permeability gas reservoirs.
Experiment
Materials
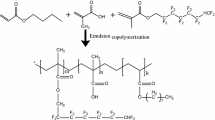
The polymer used was Zonyl8740. This DuPont product is an aqueous fluorochemical that provides water and oil repellency on porous mineral surfaces. It is soluble in water and methanol; it is chemically bond to the surface of the rock at the molecular level, allowing durable results. At room temperature, it has a density of 1.095 g/cm3 and a viscosity of less than 250 mPa s. The reservoir simulated brine with the concentration of 9,800 mg/L, and the simulated water was used. The composition is shown in Table 1.
Hexadecane is produced by Beijing Hengye company, China. Several core plugs with a diameter of about 3.7 cm sampled from the targeted gas-condensate reservoir (Changqing reservoir in China) were used in this study. As is shown in Table 2, the porosity was around 10 % and the permeability ranged from 0.13–0.23 md.
Wettability tests
The experimental work is designed to investigate wettability alteration and its effect on liquid mobility. Wettability is examined before and after treatment by contact angle measurements, capillary rise analyses, and spontaneous imbibition tests. The rock was saturated with 1.0 % Zonyl8740 solution and was aged to change the wettability at 24 °C. The aging time was about 8 h. After saturation with the chemical solution, the rock was evacuated for about an hour and was then dried in order to remove the extra liquid chemicals. A very small amount of the chemical was left on the rock surface; the surface energy decreased and the solid surface was altered to preferential gas-wettability as a result of adsorption. Liquid mobility, before and after wettability alteration, is measured by performing unsteady-state displacement tests.
Contact angles
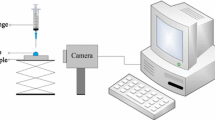
Contact angle meter SL200 B (USA Kino Industry Co.) was used to test liquid contact angles on rock surface in air at ambient temperature. Liquid contact angle measurements were conducted using the sessile drop method. Droplets of liquid were carefully dropped onto the surface of each core. Then, liquid contact angles on the rock surface were obtained. The liquid volume is about 5 × 10−3 ml. The simulated water and hexadecane were selected for measuring the contact angles of rock cores. At the same time, the images of contact angle were obtained.
Spontaneous imbibition
The experimental apparatus used to conduct the spontaneous water imbibition tests was similar to that used by Li and Horne. The schematic of apparatus is shown in Fig. 1. The core sample was hung under an electronic balance and submerged in the liquid. Change in weight of the core versus time was recorded. Spontaneous water imbibition tests were conducted to alter the wettability from preferential liquid-wetness to gas-wetness in the core sample A-1 before and after 1.0 % Zonyl8740 solution treatments. Brine was made using the compositions of the formation water. The salinity of the brine was about 98,000 mg/l. The spontaneous water imbibition into the air-saturated rock was conducted before the chemical treatment. The rate of imbibition spontaneous was calculated using the data from the electronic balance. The decrease in the imbibition rate and recovery by spontaneous imbibition of liquid after chemical treatment give the extent of wettability alteration.
The glass capillary tube rise test
The glass capillary tube rise test was conducted to examine the effect of the fluoro-copolymer emulsion on wettability alteration. The experiment procedure (Li and Firoozabadi 2000) was described as follows. Firstly, fluorochemical solutions Zonyl8740 of different concentrations were prepared with distilled water. And then, the capillary tube was aged in the solutions of various concentrations for 8 h and dried at room temperature. After the chemical treatment, the capillary tube was inserted vertically in the liquid, and then, the liquid level (h) in the capillary tube was gained, whether it rose or descended. The schematic diagram is shown in Fig. 2. According to the Young’s equation,
(where σ and ρ are surface tension and density of liquid, respectively, g is the acceleration of gravity, and r represents radius of capillary tube), the contact angle θ of the capillary tube can be computed when σ, ρ, r, and h are available. The radius of the circular capillary tube is 0.37 mm.
If the liquid in the circular capillary tube rises (h > 0), the contact angle in gas–liquid system is <90°. If there is no liquid rise (h = 0) in the capillary, the contact angle is equal to 90°. And when the liquid in the circular capillary tube goes down (h < 0), the contact angle is larger than 90°. So one may define preferential liquid-wetting when θ < 90° and preferential gas-wetting when θ > 90°. But for a capillary tube of equilateral triangle cross section, the gas–liquid interface will be flat when θ = 60°, which suggests preferential gas-wetting for θ > 60°. Actually, there is complex geometry in porous media of reservoirs. In this article, strong liquid-wetting is defined when θ < 60°, neutral gas-wetting when 60° < θ < 90°, and preferential gas-wetting when θ > 90°.
Core flooding
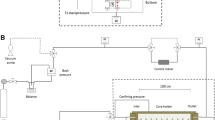
For measuring gas and liquid relative permeability before and after wettability alteration, the core was first saturated with oil (hexadecane) or the brine with a salinity of 9,800 mg/l. The absolute permeability of the core was measured. The air displacement in the core without the chemical treatment was then carried out at room temperature with a confining pressure of 500 psi, while the outlet was open to the atmosphere. Oil recovery, residual oil saturation, and gas-phase relative permeability were measured. The oil relative permeability was also calculated at low oil saturations. After the air displacement without chemical treatment, the core was cleaned, dried, and treated by 1.0 % Zonyl8740 solution. Fluorinated polymers are well known as low surface energy materials, which have oil and water repellent property (Huang et al. 2004). And the acrylic main-chains make the polymer adhere well to various materials (Zhang and Chen 2005). Following that, the chemical solution was removed from the core, and the core was saturated with oil. Air displacement was performed again. The core was then dried in order to remove the extra liquid chemicals. Oil recovery, residual oil saturation, and gas-phase relative permeability were then measured. The same experiments were repeated to measure the gas–water relative permeability after the rock was treated using the chemical solution to change the wettability. The schematic of the apparatus for core flow measurements is shown in Fig. 3.
Results and discussion
Contact angles
In order to demonstrate the wettability alteration by 1.0 % Zonyl8740 solution, droplets of water and hexadecane were placed on the surface of the rock before and after the chemical treatments (see Fig. 4). Water and hexadecane did not imbibe into the rock. Instead, it forms a ball-shape droplet. The contact angle of water and the core surface increases from 5.6° to 112.3°. The value of the measured contact angle is greater than 90°, which demonstrates that the wettability of the rock was altered to preferential gas-wetness by the chemical treatments. Figure 4b shows a droplet of hexadecane placed on the surface of the rock after the chemical treatments. The contact angle of hexadecane and the core surface increases from 35.3° to 85.7°. One can see from Fig. 4, and the value of the measured contact angle through the liquid phase that the wettability of the low-permeability rock was changed from liquid-wetness to preferential gas-wetness by the chemical treatments in the gas–liquid–rock system.
Capillary tube rise test
According to the Young equation,
where Pc is the capillary pressure, σ is the surface tension, θ is the contact angle, and r is the diameter of capillary.
If the contact angle increases, then the capillary pressure will decrease. Hence, the liquid cannot be imbibed into the capillary tube and rock core after wettability alteration to gas-wetting. Therefore, the capillary rise and spontaneous imbibition tests are used to evaluate further the effect of the emulsion on rocks surface wettability. Based on Eq. (2), the lower the capillary rise and the higher the contact angle as the surface tension of liquid is constant, indicating a deviation from strong liquid-wetting.
Figure 5 shows that liquid rise versus the concentration of Zonyl8740 solution for both the gas/oil (Hexadecane) and gas/water systems. The liquid rise in the capillary tube decreases with an increase of the concentration, and the liquid level of capillary is the lowest when the concentration reaches around 1.0 % in both gas/water and oil/gas systems. The contact angle can be calculated using Eq. (1). Figure 6 illustrates the computed contact angle versus the concentration of Zonyl8740 solution for both the gas/oil and gas/water systems. The contact angle is 25.2° in gas/oil system and 60.5° in gas/water system before treatment.
Figure 6 elucidates that the contact angle increases with the increase of the Zonyl8740 solution concentration in both the gas/oil and gas/water systems.
Imbibition and drainage
Spontaneous water imbibition tests were performed in the core samples A-2 and A-3 from the Changqing gas-condensate reservoir before and after the chemical treatments in this study. Spontaneous imbibition experiments were conducted at a room temperature to test the wettability effect of the low-permeability rock sampled from liquid-wetness to gas-wetness.
Figure 7 plots the gas recovery by both oil (hexadecane) and water spontaneous imbibitions in an air-saturated core before and after treatment. The gas recovery is represented by the fraction of gas originally in-place (GOIP). One can see from Fig. 7 that the final recovery of the reservoir rock (A-2) by spontaneous water imbibition was about 75.6 % without the chemical treatment. After the chemical treatment, the amount of water imbibed into the reservoir rock was almost zero. The final recovery of the reservoir rock (A-3) by spontaneous oil imbibition was about 46.9 % without the chemical treatment. After the chemical treatment, the amount of oil imbibed into the reservoir rock was almost zero too. This demonstrates the wettability alteration and a brief explanation are given in the following. There are only two types of forces, gravity and capillary forces, that govern the spontaneous water imbibition shown in Fig. 1. In this case, capillary pressure is the driving force, while gravity is the resistance force. Gravity force did not change during the spontaneous water imbibition with and without the chemical treatments. So the only possibility was because of the change in capillary pressure to cause the reduction in recovery by spontaneous liquid imbibition. A simple definition of capillary pressure is adopted here:
where Pc is the capillary pressure, k and ϕ are permeability and porosity, respectively; δ is the surface tension and θp pseudo contact angle. We found that there was no change in surface tension and almost no change in porosity and permeability by the chemical treatment. Based on the above analysis and according to Eq. 2, it was the change in wettability represented by the contact angle to cause the reduction in recovery by spontaneous liquid imbibition shown in Fig. 7. Therefore, the results shown in Fig. 7 demonstrate that the wettability of the very low-permeability rock alters from liquid-wetness to gas-wetness significantly.
A significant decrease in liquid imbibition rate with treatment is observed from Fig. 8. The spontaneous imbibition is related to wettability and gas recovery by spontaneous liquid imbibition decreases when the rock wettability changes from liquid-wetting to preferential gas-wetting. Therefore, these results demonstrate that the wettability of reservoirs rock in both gas/water and gas/oil systems has been altered from strong liquid-wetting to intermediate gas-wetting by the chemical treatment. These results are consistent with our previous contact angle measurement and capillary rise tests.
Core flooding test
Effect of wettability alteration on relative permeability
The gas and liquid relative permeability by gas flooding in the rock were calculated using the experimental data measured before and after the wettability alteration. The relative permeabilities were calculated by the JBN method (Johnson et al. 1959). Figure 9 shows the values of gas and water or oil (hexadecane) relative permeability measured in the core sample A-4 using an unsteady-state displacement approach before and after wettability alteration by the chemical treatments. After core flooding tests, a significant effect of wettability alteration on both gas and oil relative permeabilities was observed in Fig. 9a. Both the gas and water phase relative permeability increased after the wettability was altered from liquid-wetness to gas-wetness. The residual oil saturation by gas flooding decreased from 0.418 to 0.361 PV (PV = pore volume); the gas-phase relative permeability at the residual oil saturation increased about two times because of the wettability alteration from liquid-wetness to gas-wetness.
Gas and water relative permeabilities were measured in the core sample A-5 using the same unsteady-state displacement approach before and after wettability alteration by the chemical treatments. The effect of wettability alteration on gas and water relative permeabilities shows a trend similar to that for gas and oil relative permeabilities (see Fig. 9b). Residual water saturation decreased from 0.45 to 0.35 PV. Water saturation at the cross point, krg = krw, for treated cores was less than 0.4 PV, implying gas-wetting. This result is in line with the spontaneous imbibition measurements. These results imply that gas well deliverability may increase substantially when wettability is altered to intermediate gas-wetting.
Effect of wettability alteration on recovery
Figure 10 plots the values of recovery by gas flooding before and after the wettability alteration by the chemical treatments. The water recovery (see Fig. 10a) by gas flooding increased significantly after the wettability alteration. The final water recovery by gas flooding after wettability alteration increased from 50.5 to 72.9 %. The effect of wettability alteration on oil recovery shows a trend similar to that of water recovery (see Fig. 10b). The final oil (hexadecane) recovery by gas flooding after wettability alteration increased from 60.9 to 80.6 %. In summarizing, the experimental results shown above demonstrate that the effects of wettability alteration from liquid-wetness to gas-wetness on gas–water relative permeability and recovery are very significant in the rock with a low permeability.
Thermal stability
The thermal stability of the chemical treatment is very important in this study because the Changqing gas-condensate reservoir has a temperature of about 170 °C. To meet the requirement of high reservoir temperature, the thermal stability of the chemical Zonyl8740 was tested and verified. The experimental procedure and results are described briefly. The solution of Zonyl8740 solution (100 ml) was filled in a stainless steel cylinder and was pressurized to 5 MPa using nitrogen. Then, the cylinder with the solution was sealed and was put in an oven in which the temperature was raised gradually to 170 °C. The experimental results demonstrated that the chemical solution could still change the wettability of the low-permeability rock after 48 h high temperature aging.
In summarizing, the experimental results demonstrate that the effects of wettability alteration from liquid-wetness to gas-wetness on gas–liquid relative permeability and recovery are very significant in the rock with a low permeability of less than 1.0 md. One can expect that the wettability alteration from water-wetness to gas-wetness may enhance the gas well deliverability and the gas production notably in gas-condensate reservoirs.
Conclusions
-
1.
The wettability of gas–liquid–rock systems can be altered from strong liquid-wetting to intermediate gas-wetting by Zonyl8740. The capillary rise test ensured the effectiveness of wettability alteration by Zonyl8740, and the result was in good agreement with contact angle measurement. It was evidenced by the spontaneous imbibition tests that Zonyl8740 could effectively suppress the imbibition of water and oil into rock.
-
2.
Experimental results demonstrated that the relative permeabilities of both the gas and the liquid phases were increased significantly after the wettability alteration to preferential gas-wetness. The residual liquid saturation was decreased, and the gas production was enhanced greatly due to the wettability alteration. These results imply that gas well deliverability may increase substantially when wettability is altered to intermediate gas-wetting.
-
3.
Note that the wettability alteration to gas-wetness may also be applied in drilling and completion to reduce the water loss to tight gas formation, in water shutoff in tight gas reservoirs with bottom water, and acid-fracturing to contain secondary formation damage in low-permeability gas reservoirs.
References
Allen FH, Roe RP (1950) Performance characteristics of a volumetric condensate reservoir. Trans AIME 189:83
Barnum RS (1995) Gas-condensate reservoir behavior: productivity and recovery reduction due to condensation, paper SPE 30767 presented at the 1995 Annual Technical Conference and Exhibition, Dallas, 22–25 October
Boom W, Wit K, Zeelenberg JPW, Weeda HC, Maas JG (1996) On the use of model experiments for assessing improved gas-condensate mobility under near-wellbore flow conditions, paper SPE 36714 presented at the 1996 SPE annual technical conference and exhibition, Denver, Colorado, Oct. 6–9
Fahes M, Firoozabadi A (2005) Wettability alteration to intermediate gas wetting in gas condensate reservoirs at high temperatures, paper SPE 96184 presented at the SPE annual technical conference and exhibition held in Dallas, Texas, CA, 9–12 October 2005
Holditch SA (1979) Factors affecting water blocking and gas flow from hydraulically fractured gas wells, paper SPE 7561, SPE AIME, December 1979
Huang PY, Chao YC, Liao YT (2004) Preparation of fluoroacrylate nanocopolymer by miniemulsion polymerization used in textile finishing. J Appl Polym Sci 94:1466–1472
Johnson EF, Bossler DP, Naumann VO (1959) Calculation of relative permeability from displacement experiments. Trans AIME 216:370–372
Li K, Firoozabadi A (2000) Phenomenological modeling of critical-condensate saturation and relative permeabilities in gas-condensate systems, paper SPE 56014 available from SPE, Richardson, Texas
Mahadevan J, Sharma MM (2003) Clean-up of water blocks in low permeability formations, paper SPE 84216 presented at the SPE annual technical conference and exhibition held in Denver, Colorado, USA, 5–8 October 2003
Tang G, Firoozabadi A (2003) Wettability alteration to intermediate gas wetting in porous media at elevated temperatures. Transp Porous Media 52:185–211
Tannich JD (1975) Liquid removal from hydraulically fractured wells. J Pet Tech 1309–1317
Wagner OR, Leach RO (1958) Improving oil displacement efficiency by wettability adjustment, paper SPE 1101-G presented at the 1958 Annual Meeting, Houston, 5–8 October
Walker JG, Pope GA, Sharma MM, Wang P (2005) Use of solvents to improve the productivity of gas condensate wells, paper SPE 62935 presented at the SPE Annual Technical Conference and Exhibition held in Dallas, Texas, CA, 1–4 October 2005
Zhang CC, Chen YJ (2005) Investigation of fluorinated polyacrylate latex with coreshell structure. Polym Int 54:1027–1033
Zuluaga E, Monsalve JC (2003) Water vaporization in gas reservoirs, paper SPE 84829 presented at the SPE Eastern Regional/AAPG Eastern Section Joint Meeting held in Pittsburgh, Pennsylvania, USA, 6–10 September 2003
Acknowledgments
This work is supported by the national science foundation of innovative research group project (Project NO. 51221003), the National Science and Technology Major Project(Project NO. 2011ZX05050)and the National Science Fund (Project NO.51074173).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Zhang, S., Jiang, GC., Wang, L. et al. Wettability alteration to intermediate gas-wetting in low-permeability gas-condensate reservoirs. J Petrol Explor Prod Technol 4, 301–308 (2014). https://doi.org/10.1007/s13202-014-0119-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13202-014-0119-9