-
PDF
- Split View
-
Views
-
Cite
Cite
JÜRGEN KONZETT, YINGWEI FEI, Transport and Storage of Potassium in the Earth’s Upper Mantle and Transition Zone: an Experimental Study to 23 GPa in Simplified and Natural Bulk Compositions, Journal of Petrology, Volume 41, Issue 4, April 2000, Pages 583–603, https://doi.org/10.1093/petrology/41.4.583
Close - Share Icon Share
Abstract
We have investigated the stability and composition of potassium amphibole and its high-pressure breakdown product phase X in synthetic peralkaline and subalkaline KNCMASH (K2O–Na2O–CaO–MgO–Al2O3–SiO2–H2O) and natural KLB-1 peridotite bulk compositions between 10 and 23 GPa at 800–1800°C. In the KNCMASH system, potassium amphibole reaches its upper pressure stability limit at 13–15 GPa at ≤1400°C. In the natural KLB-1 bulk composition, potassium amphibole breaks down between 12 and 13 GPa at 1200°C. Phase X is a hydrous potassium–magnesium silicate with variable stoichiometry, a general formula K2–xMg2Si2O7Hx with x = 0–1, and a maximum possible H2O content of 3·5 wt %. Electron microprobe analytical totals suggest H2O contents of ∼1–2 wt % and a decrease in H2O contents with increasing pressure. In both KNCMASH and KLB-1 systems, phase X coexists with Mg2SiO4 + garnet + high-Ca clinopyroxene + low-Ca clinopyroxene ± fluid. Phase X breaks down between 20 and 23 GPa at 1500–1700°C to form K-hollandite + γ-Mg2SiO4 + majorite + Ca-perovskite + fluid. The upper temperature stability limit of phase X was located in the subalkaline KNCMASH system between 1400 and 1600°C at 14 GPa and at >1700°C at 20 GPa, the latter being at least 200°C above an average current mantle adiabat. Thus, phase X could store and transport both water and potassium not only in subduction zone settings, but also in convecting mantle down to the transition zone–lower-mantle boundary. Phase X would also be an eminently suitable host for Rb, Cs, Ba or Pb.
INTRODUCTION
The hydrous potassic phases (HPP) phlogopite and K-amphibole are major storage sites for potassium in the Earth’s upper mantle. Both host the incompatible trace elements with large ionic radii (Rb, Ba or Pb), which can occupy large and highly coordinated lattice positions in phlogopite and K-amphibole (Basu, 1978; Foley et al., 1995; Ionov et al., 1997). Phlogopite is an important component in sources of kimberlites, lamproites, lamprophyres and K-rich basaltic rocks (e.g. Wilkinson & LeMaitre, 1986; Esperança & Holloway, 1987; Rogers, 1992; Mitchell, 1995, and references therein; Sato, 1997). K-richterite has been suggested as a possible source of K, Ti and high field strength elements (HFSE) for kimberlites and ultrapotassic rocks (Foley, 1992; Taylor et al., 1994). Phlogopite is stable in Al-rich metasedimentary and peridotitic bulk compositions. In Al-rich bulk compositions phlogopite breaks down at relatively low P (and T) to form phengite ± K-feldspar and possibly K,Mg-rich fluid (Massonne & Schreyer, 1989; Massonne, 1992). In peridotitic bulk compositions, phlogopite is stable to at least 6 GPa and 1100°C with orthopyroxene + clinopyroxene + olivine (Konzett & Ulmer, 1999; see also Wendlandt & Eggler, 1980; Mengel & Green, 1989, for experiments at lower P) and to at least 12 GPa and 1350°C with phlogopite + clinopyroxene (Luth, 1997). Phlogopite is stable above the solidus of natural lherzolite at P ≤ 3 GPa (Wendlandt & Eggler, 1980). Experiments with phlogopite + enstatite (Sato et al., 1997) suggest above-solidus stability of phlogopite through continuous melting reactions between 4 and 8 GPa that form olivine + pyrope. Although it is difficult to distinguish hydrous melts from fluids at high P, K-richterite is likely to be unstable above the solidus in either subalkaline or peralkaline bulk compositions (Konzett et al., 1997; Konzett & Ulmer, 1999). However, K-richterite probably controls the P–T location of the solidus by incongruent melting (Gilbert & Briggs, 1974; Foley, 1991).
K-amphibole with a composition close to KNaCaMg5Si8O22(OH)2 can form as a high-P breakdown product of phlogopite in peridotitic bulk compositions at P > 6 GPa (Trønnes et al., 1988; Luth, 1997; Konzett & Ulmer, 1999). In Na-free systems the K-amphibole KKCaMg5Si8O22(OH)2 is present (Sudo & Tatsumi, 1990; Luth, 1997; Inoue et al., 1998; Yang et al., 1999). In peralkaline bulk compositions [mica–amphibole–rutile–ilmenite–diopside (MARID), lamproites] phase relations from natural rocks and from high-P experiments suggest a continuous stability of K-amphibole + phlogopite from 0·1 MPa to at least 8·5 GPa (Mitchell & Bergman, 1991, and references therein; Konzett et al., 1997).
Experimental studies of the KCMSH and KCMASH systems (Luth, 1997; Inoue et al., 1998) show that K-amphibole breaks down at high pressures to a hydrous K-rich silicate that is capable of transporting alkalis and water to even greater depths than does amphibole. The structure, stoichiometry and compositional variability of this phase—termed phase X (Luth, 1995)—are still unknown and its stability field is poorly constrained. The aims of our study are to (1) better constrain the stability field of phase X, especially its high-pressure stability limit; and (2) determine the chemical variability of phase X and coexisting phases. This will permit us to assess the potential of phase X as a storage site for water and alkalis in the mantle transition zone and to trace mechanisms of potassium and water recycling into the mantle.
COMPOSITION OF STARTING MATERIALS
Our subalkaline and peralkaline starting materials are mixes of high purity (≥99·95% purity) synthetic oxides or silicates and carbonates, and cover the full range of bulk compositions that can stabilize K-amphibole and its breakdown products (Table 1). They represent peridotitic and MARID-type (Dawson & Smith 1977) or lamproitic bulk composition, respectively. The phase relations of these bulk compositions at P < 10 GPa have been described by Konzett et al. (1997) and Konzett & Ulmer (1999). A simplified K2O–Na2O–CaO–MgO–Al2O3–SiO2–H2O (KNCMASH) system was examined to avoid the potential influence of fO2 on phase relations in an Fe-bearing system at very high pressures as a result of stabilization of phases by Fe3+ partitioning. Despite this simplification, the system is still complex enough to permit all important exchange reactions (MgSiAl−2, NaSiCa−1Al−1, CaMg−1, KNa−1) among the silicate phases. Additional experiments were conducted with KLB-1 peridotite (Takahashi, 1986) doped with 10 wt % synthetic K-richterite, to locate the amphibole → phase X transition in a natural peridotitic bulk composition.
Compositions of synthetic and natural starting materials
| KNCMASH | KNCMASH | KLB-1 | KLB-1 + | |
| subalk bulk | peralk bulk | 10 wt % Kr | ||
| SiO2 | 47·28 | 47·61 | 44·48 | 45·67 |
| TiO2 | — | — | 0·16 | 0·15 |
| Al2O3 | 11·50 | 6·67 | 3·59 | 3·27 |
| Cr2O3 | — | — | 0·31 | 0·28 |
| FeO* | — | — | 8·10 | 7·37 |
| Fe2O3 | — | — | — | — |
| MnO | — | — | 0·12 | 0·11 |
| MgO | 27·26 | 23·79 | 39·22 | 37·87 |
| CaO | 6·58 | 7·61 | 3·44 | 3·74 |
| Na2O | 1·22 | 1·94 | 0·30 | 0·69 |
| K2O | 4·94 | 7·62 | 0·02 | 0·40 |
| P2O5 | — | — | 0·03 | 0·03 |
| NiO | — | — | 0·25 | 0·23 |
| H2O | 1·22 | 4·76 | — | 0·20 |
| ∑ | 100·00 | 100·00 | 100·02 | 100·01 |
| PI | 0·64 | 1·72 | 0·48 | |
| K/Na | 2·66 | 2·58 | 0·38 |
| KNCMASH | KNCMASH | KLB-1 | KLB-1 + | |
| subalk bulk | peralk bulk | 10 wt % Kr | ||
| SiO2 | 47·28 | 47·61 | 44·48 | 45·67 |
| TiO2 | — | — | 0·16 | 0·15 |
| Al2O3 | 11·50 | 6·67 | 3·59 | 3·27 |
| Cr2O3 | — | — | 0·31 | 0·28 |
| FeO* | — | — | 8·10 | 7·37 |
| Fe2O3 | — | — | — | — |
| MnO | — | — | 0·12 | 0·11 |
| MgO | 27·26 | 23·79 | 39·22 | 37·87 |
| CaO | 6·58 | 7·61 | 3·44 | 3·74 |
| Na2O | 1·22 | 1·94 | 0·30 | 0·69 |
| K2O | 4·94 | 7·62 | 0·02 | 0·40 |
| P2O5 | — | — | 0·03 | 0·03 |
| NiO | — | — | 0·25 | 0·23 |
| H2O | 1·22 | 4·76 | — | 0·20 |
| ∑ | 100·00 | 100·00 | 100·02 | 100·01 |
| PI | 0·64 | 1·72 | 0·48 | |
| K/Na | 2·66 | 2·58 | 0·38 |
KLB-1 analysis, Takahashi (1986); composition of synthetic K-richterite (Kr) (average of 10 analyses): SiO2 57·22(20); MgO 24·20(22); CaO 6·71(19); Na2O 4·61(09); K2O 4·16(11); analytical procedure is as given by Konzett & Ulmer (1999); PI (peralkalinity index) = molar (K2O + Na2O)/Al2O3.
Compositions of synthetic and natural starting materials
| KNCMASH | KNCMASH | KLB-1 | KLB-1 + | |
| subalk bulk | peralk bulk | 10 wt % Kr | ||
| SiO2 | 47·28 | 47·61 | 44·48 | 45·67 |
| TiO2 | — | — | 0·16 | 0·15 |
| Al2O3 | 11·50 | 6·67 | 3·59 | 3·27 |
| Cr2O3 | — | — | 0·31 | 0·28 |
| FeO* | — | — | 8·10 | 7·37 |
| Fe2O3 | — | — | — | — |
| MnO | — | — | 0·12 | 0·11 |
| MgO | 27·26 | 23·79 | 39·22 | 37·87 |
| CaO | 6·58 | 7·61 | 3·44 | 3·74 |
| Na2O | 1·22 | 1·94 | 0·30 | 0·69 |
| K2O | 4·94 | 7·62 | 0·02 | 0·40 |
| P2O5 | — | — | 0·03 | 0·03 |
| NiO | — | — | 0·25 | 0·23 |
| H2O | 1·22 | 4·76 | — | 0·20 |
| ∑ | 100·00 | 100·00 | 100·02 | 100·01 |
| PI | 0·64 | 1·72 | 0·48 | |
| K/Na | 2·66 | 2·58 | 0·38 |
| KNCMASH | KNCMASH | KLB-1 | KLB-1 + | |
| subalk bulk | peralk bulk | 10 wt % Kr | ||
| SiO2 | 47·28 | 47·61 | 44·48 | 45·67 |
| TiO2 | — | — | 0·16 | 0·15 |
| Al2O3 | 11·50 | 6·67 | 3·59 | 3·27 |
| Cr2O3 | — | — | 0·31 | 0·28 |
| FeO* | — | — | 8·10 | 7·37 |
| Fe2O3 | — | — | — | — |
| MnO | — | — | 0·12 | 0·11 |
| MgO | 27·26 | 23·79 | 39·22 | 37·87 |
| CaO | 6·58 | 7·61 | 3·44 | 3·74 |
| Na2O | 1·22 | 1·94 | 0·30 | 0·69 |
| K2O | 4·94 | 7·62 | 0·02 | 0·40 |
| P2O5 | — | — | 0·03 | 0·03 |
| NiO | — | — | 0·25 | 0·23 |
| H2O | 1·22 | 4·76 | — | 0·20 |
| ∑ | 100·00 | 100·00 | 100·02 | 100·01 |
| PI | 0·64 | 1·72 | 0·48 | |
| K/Na | 2·66 | 2·58 | 0·38 |
KLB-1 analysis, Takahashi (1986); composition of synthetic K-richterite (Kr) (average of 10 analyses): SiO2 57·22(20); MgO 24·20(22); CaO 6·71(19); Na2O 4·61(09); K2O 4·16(11); analytical procedure is as given by Konzett & Ulmer (1999); PI (peralkalinity index) = molar (K2O + Na2O)/Al2O3.
EXPERIMENTAL AND ANALYTICAL TECHNIQUES
Experiments (Table 2) were performed with Walker-type and split-sphere MA-8 multianvil devices at the Geophysical Laboratory (GL) and at the Bayerisches Geoinstitut (BG), respectively, using prefabricated pyrophyllite gaskets and MgO octahedra. Assembly sizes and furnace materials are as follows: GL: 10/5 (10 mm edge length of octahedra/5 mm truncated edge length of WC cubes) and 8/3 assemblies to 15 and 23 GPa using Re heaters; BG: 14/7 assemblies using stepped LaCrO3 heaters. Pre-dried starting materials were placed in 1·55 mm or 1·00 mm (GL, for 8/3 assemblies) outer diameter Pt100 capsules and welded shut immediately. For the KLB-1 starting material, an additional inner graphite capsule (approximate dimensions after runs: wall thickness 300 μm, bottom and lid 200 μm) was added. To minimize T gradients and phase separation as a result of thermal diffusion, the length of experimental charges ranged between 200 and 600 μm. T gradients were ∼20°C/100 μm for 8/3 assemblies and <10°C/100 μm for 10/5 assemblies (Bertka & Fei, 1997, and unpublished data, 1999). Temperatures were measured with W3%Re–W25%Re thermocouples without correcting for the pressure effect on e.m.f. Both pressure and temperature were computer controlled during the runs. Detailed descriptions of the GL and BG experimental and calibration procedures have been given by Bertka & Fei (1997) and Rubie et al. (1993), respectively.
Summary of experimental run conditions and run products
| Run | Bulk | Assembly | Furnace | P | T | Run time | Phases observed |
| (GPa) | (°C) | ||||||
| Ma88B | per | 14/8 | LaCr | 13·0 | 1100 | 07h30 | Kr+ga+Mg2SiO4+hiCapx |
| Ma91B | per | 14/8 | LaCr | 14·0 | 1100 | 09h25 | Kr+pX+ga+Mg2SiO4+hiCapx |
| Ma92B | per | 14/8 | LaCr | 15·0 | 1100 | 09h40 | pX+ga+Mg2SiO4+hiCapx+loCapx |
| Ma102M | per | 14/8 | LaCr | 18·0 | 1300 | 09h45 | pX+ga+Mg2SiO4+hiCapx+Q |
| Ma104M | per | 14/8 | LaCr | 13·0 | 1400 | 10h00 | ga+hiCapx+Mg2SiO4+Q |
| JKW7 | per | 10/5 | Re | 14·0 | 1300 | 07h00 | pX+ga+hiCapx+Mg2SiO4+loCapx+Q |
| JKW9 | per | 10/5 | Re | 12·0 | 1300 | 06h08 | Kr+pX+ga+hiCapx+Mg2SiO4+Q |
| JKW13 | per | 10/5 | Re | 10·0 | 1200 | 12h00 | Kr+pyr+phl+ga+hiCapx+Mg2SiO4+Q |
| JKW14 | per | 10/5 | Re | 14·0 | 1200 | 12h00 | Kr+pX+ga+Mg2SiO4+hiCapx+ |
| loCapx+Q | |||||||
| JKW15 | per | 10/5 | Re | 10·0 | 1300 | 48h00 | Kr+pyr+phl+ga+hiCapx+Mg2SiO4+Q |
| JKW16 | per | 8/3 | Re | 20·0 | 1300 | 07h00 | pX+ Mg2SiO4+ga+K-holl+Ca-perov |
| JKW17 | per | 10/5 | Re | 11·0 | 1300 | 48h00 | Kr+hiCapx+ga+Mg2SiO4+Q |
| JKW18 | per | 10/5 | Re | 10·0 | 1350 | 08h00 | Kr+phl+ga+hiCapx+Mg2SiO4+Q |
| JKW19 | per | 10/5 | Re | 10·0 | 1100 | 10h00 | Kr+phl+ga+hiCapx+Mg2SiO4+Q |
| JKW25 | per | 10/5 | Re | 15·0 | 900 | 36h00 | Kr+pX+pyr+hiCapx+loCapx+ga+ |
| Mg2SiO4 | |||||||
| Ma95sB | sub | 14/8 | LaCr | 10·0 | 1100 | 10h00 | Mg2SiO4+hiCapx+ga+Kr |
| Ma94sB | sub | 14/8 | LaCr | 15·0 | 1100 | 09h48 | Mg2SiO4+hiCapx+loCapx+ga+pX |
| JKW29 | sub | 10/5 | Re | 14·0 | 1100 | 11h16 | Mg2SiO4+hiCapx+ga+pX |
| JKW41 | sub | 10/5 | Re | 13·0 | 1300 | 11h45 | Mg2SiO4+hiCapx+loCapx+ga+Kr |
| JKW33 | sub | 10/5 | Re | 14·0 | 1400 | 12h00 | Mg2SiO4+hiCapx+loCapx+ga+pX |
| JKW34 | sub | 10/5 | Re | 14·0 | 900 | 48h00 | hiCapx+loCapx +ga+phase E+pX |
| JKW30 | sub | 10/5 | Re | 13·0 | 1100 | 30h00 | Mg2SiO4+hiCapx+loCapx+ga+Kr |
| JKW47 | sub | 8/3 | Re | 20·0 | 1500 | 05h00 | pX+Mg2SiO4+ga+K-holl+Ca-perov |
| JKW54 | sub | 8/3 | Re | 23·0 | 1700 | 03h00 | Mg2SiO4+ga+K-holl+Ca-perov+Q |
| JKW61 | sub | 8/3 | Re | 23·0 | 1500 | 12h00 | Mg2SiO4+ga+K-holl+Ca-perov+Q |
| JKW64 | sub | 8/3 | Re | 20·0 | 1600 | 06h00 | Mg2SiO4+ga+pX+Ca-perov+Q |
| JKW66 | sub | 8/3 | Re | 14·0 | 1600 | 08h00 | ga+loCapx+hiCapX+Q |
| JKW67 | sub | 8/3 | Re | 20·0 | 1800* | 03h00 | ga+Ca-perov+pX+Q |
| JKW63 | KLB | 10/5 | Re | 12·0 | 1200 | 72h00 | ga+loCapx+hiCapx+Mg2SiO4+Kr |
| JKW69 | KLB | 10/5 | Re | 14·0 | 1200 | 48h00 | ga+loCapx+hiCapX+Mg2SiO4+pX |
| JKW70 | KLB | 10/5 | Re | 13·0 | 1200 | 72h00 | ga+loCapx+hiCapX+ol |
| Run | Bulk | Assembly | Furnace | P | T | Run time | Phases observed |
| (GPa) | (°C) | ||||||
| Ma88B | per | 14/8 | LaCr | 13·0 | 1100 | 07h30 | Kr+ga+Mg2SiO4+hiCapx |
| Ma91B | per | 14/8 | LaCr | 14·0 | 1100 | 09h25 | Kr+pX+ga+Mg2SiO4+hiCapx |
| Ma92B | per | 14/8 | LaCr | 15·0 | 1100 | 09h40 | pX+ga+Mg2SiO4+hiCapx+loCapx |
| Ma102M | per | 14/8 | LaCr | 18·0 | 1300 | 09h45 | pX+ga+Mg2SiO4+hiCapx+Q |
| Ma104M | per | 14/8 | LaCr | 13·0 | 1400 | 10h00 | ga+hiCapx+Mg2SiO4+Q |
| JKW7 | per | 10/5 | Re | 14·0 | 1300 | 07h00 | pX+ga+hiCapx+Mg2SiO4+loCapx+Q |
| JKW9 | per | 10/5 | Re | 12·0 | 1300 | 06h08 | Kr+pX+ga+hiCapx+Mg2SiO4+Q |
| JKW13 | per | 10/5 | Re | 10·0 | 1200 | 12h00 | Kr+pyr+phl+ga+hiCapx+Mg2SiO4+Q |
| JKW14 | per | 10/5 | Re | 14·0 | 1200 | 12h00 | Kr+pX+ga+Mg2SiO4+hiCapx+ |
| loCapx+Q | |||||||
| JKW15 | per | 10/5 | Re | 10·0 | 1300 | 48h00 | Kr+pyr+phl+ga+hiCapx+Mg2SiO4+Q |
| JKW16 | per | 8/3 | Re | 20·0 | 1300 | 07h00 | pX+ Mg2SiO4+ga+K-holl+Ca-perov |
| JKW17 | per | 10/5 | Re | 11·0 | 1300 | 48h00 | Kr+hiCapx+ga+Mg2SiO4+Q |
| JKW18 | per | 10/5 | Re | 10·0 | 1350 | 08h00 | Kr+phl+ga+hiCapx+Mg2SiO4+Q |
| JKW19 | per | 10/5 | Re | 10·0 | 1100 | 10h00 | Kr+phl+ga+hiCapx+Mg2SiO4+Q |
| JKW25 | per | 10/5 | Re | 15·0 | 900 | 36h00 | Kr+pX+pyr+hiCapx+loCapx+ga+ |
| Mg2SiO4 | |||||||
| Ma95sB | sub | 14/8 | LaCr | 10·0 | 1100 | 10h00 | Mg2SiO4+hiCapx+ga+Kr |
| Ma94sB | sub | 14/8 | LaCr | 15·0 | 1100 | 09h48 | Mg2SiO4+hiCapx+loCapx+ga+pX |
| JKW29 | sub | 10/5 | Re | 14·0 | 1100 | 11h16 | Mg2SiO4+hiCapx+ga+pX |
| JKW41 | sub | 10/5 | Re | 13·0 | 1300 | 11h45 | Mg2SiO4+hiCapx+loCapx+ga+Kr |
| JKW33 | sub | 10/5 | Re | 14·0 | 1400 | 12h00 | Mg2SiO4+hiCapx+loCapx+ga+pX |
| JKW34 | sub | 10/5 | Re | 14·0 | 900 | 48h00 | hiCapx+loCapx +ga+phase E+pX |
| JKW30 | sub | 10/5 | Re | 13·0 | 1100 | 30h00 | Mg2SiO4+hiCapx+loCapx+ga+Kr |
| JKW47 | sub | 8/3 | Re | 20·0 | 1500 | 05h00 | pX+Mg2SiO4+ga+K-holl+Ca-perov |
| JKW54 | sub | 8/3 | Re | 23·0 | 1700 | 03h00 | Mg2SiO4+ga+K-holl+Ca-perov+Q |
| JKW61 | sub | 8/3 | Re | 23·0 | 1500 | 12h00 | Mg2SiO4+ga+K-holl+Ca-perov+Q |
| JKW64 | sub | 8/3 | Re | 20·0 | 1600 | 06h00 | Mg2SiO4+ga+pX+Ca-perov+Q |
| JKW66 | sub | 8/3 | Re | 14·0 | 1600 | 08h00 | ga+loCapx+hiCapX+Q |
| JKW67 | sub | 8/3 | Re | 20·0 | 1800* | 03h00 | ga+Ca-perov+pX+Q |
| JKW63 | KLB | 10/5 | Re | 12·0 | 1200 | 72h00 | ga+loCapx+hiCapx+Mg2SiO4+Kr |
| JKW69 | KLB | 10/5 | Re | 14·0 | 1200 | 48h00 | ga+loCapx+hiCapX+Mg2SiO4+pX |
| JKW70 | KLB | 10/5 | Re | 13·0 | 1200 | 72h00 | ga+loCapx+hiCapX+ol |
Abbreviations of mineral phases are given in Table A1; per, peralkaline KNCMASH system; sub, subalkaline KNCMASH system; KLB = KLB-1 + 10 wt % synthetic K-richterite.
*Capsule slightly off-center, therefore actual T was probably not higher than 1700°C.
Summary of experimental run conditions and run products
| Run | Bulk | Assembly | Furnace | P | T | Run time | Phases observed |
| (GPa) | (°C) | ||||||
| Ma88B | per | 14/8 | LaCr | 13·0 | 1100 | 07h30 | Kr+ga+Mg2SiO4+hiCapx |
| Ma91B | per | 14/8 | LaCr | 14·0 | 1100 | 09h25 | Kr+pX+ga+Mg2SiO4+hiCapx |
| Ma92B | per | 14/8 | LaCr | 15·0 | 1100 | 09h40 | pX+ga+Mg2SiO4+hiCapx+loCapx |
| Ma102M | per | 14/8 | LaCr | 18·0 | 1300 | 09h45 | pX+ga+Mg2SiO4+hiCapx+Q |
| Ma104M | per | 14/8 | LaCr | 13·0 | 1400 | 10h00 | ga+hiCapx+Mg2SiO4+Q |
| JKW7 | per | 10/5 | Re | 14·0 | 1300 | 07h00 | pX+ga+hiCapx+Mg2SiO4+loCapx+Q |
| JKW9 | per | 10/5 | Re | 12·0 | 1300 | 06h08 | Kr+pX+ga+hiCapx+Mg2SiO4+Q |
| JKW13 | per | 10/5 | Re | 10·0 | 1200 | 12h00 | Kr+pyr+phl+ga+hiCapx+Mg2SiO4+Q |
| JKW14 | per | 10/5 | Re | 14·0 | 1200 | 12h00 | Kr+pX+ga+Mg2SiO4+hiCapx+ |
| loCapx+Q | |||||||
| JKW15 | per | 10/5 | Re | 10·0 | 1300 | 48h00 | Kr+pyr+phl+ga+hiCapx+Mg2SiO4+Q |
| JKW16 | per | 8/3 | Re | 20·0 | 1300 | 07h00 | pX+ Mg2SiO4+ga+K-holl+Ca-perov |
| JKW17 | per | 10/5 | Re | 11·0 | 1300 | 48h00 | Kr+hiCapx+ga+Mg2SiO4+Q |
| JKW18 | per | 10/5 | Re | 10·0 | 1350 | 08h00 | Kr+phl+ga+hiCapx+Mg2SiO4+Q |
| JKW19 | per | 10/5 | Re | 10·0 | 1100 | 10h00 | Kr+phl+ga+hiCapx+Mg2SiO4+Q |
| JKW25 | per | 10/5 | Re | 15·0 | 900 | 36h00 | Kr+pX+pyr+hiCapx+loCapx+ga+ |
| Mg2SiO4 | |||||||
| Ma95sB | sub | 14/8 | LaCr | 10·0 | 1100 | 10h00 | Mg2SiO4+hiCapx+ga+Kr |
| Ma94sB | sub | 14/8 | LaCr | 15·0 | 1100 | 09h48 | Mg2SiO4+hiCapx+loCapx+ga+pX |
| JKW29 | sub | 10/5 | Re | 14·0 | 1100 | 11h16 | Mg2SiO4+hiCapx+ga+pX |
| JKW41 | sub | 10/5 | Re | 13·0 | 1300 | 11h45 | Mg2SiO4+hiCapx+loCapx+ga+Kr |
| JKW33 | sub | 10/5 | Re | 14·0 | 1400 | 12h00 | Mg2SiO4+hiCapx+loCapx+ga+pX |
| JKW34 | sub | 10/5 | Re | 14·0 | 900 | 48h00 | hiCapx+loCapx +ga+phase E+pX |
| JKW30 | sub | 10/5 | Re | 13·0 | 1100 | 30h00 | Mg2SiO4+hiCapx+loCapx+ga+Kr |
| JKW47 | sub | 8/3 | Re | 20·0 | 1500 | 05h00 | pX+Mg2SiO4+ga+K-holl+Ca-perov |
| JKW54 | sub | 8/3 | Re | 23·0 | 1700 | 03h00 | Mg2SiO4+ga+K-holl+Ca-perov+Q |
| JKW61 | sub | 8/3 | Re | 23·0 | 1500 | 12h00 | Mg2SiO4+ga+K-holl+Ca-perov+Q |
| JKW64 | sub | 8/3 | Re | 20·0 | 1600 | 06h00 | Mg2SiO4+ga+pX+Ca-perov+Q |
| JKW66 | sub | 8/3 | Re | 14·0 | 1600 | 08h00 | ga+loCapx+hiCapX+Q |
| JKW67 | sub | 8/3 | Re | 20·0 | 1800* | 03h00 | ga+Ca-perov+pX+Q |
| JKW63 | KLB | 10/5 | Re | 12·0 | 1200 | 72h00 | ga+loCapx+hiCapx+Mg2SiO4+Kr |
| JKW69 | KLB | 10/5 | Re | 14·0 | 1200 | 48h00 | ga+loCapx+hiCapX+Mg2SiO4+pX |
| JKW70 | KLB | 10/5 | Re | 13·0 | 1200 | 72h00 | ga+loCapx+hiCapX+ol |
| Run | Bulk | Assembly | Furnace | P | T | Run time | Phases observed |
| (GPa) | (°C) | ||||||
| Ma88B | per | 14/8 | LaCr | 13·0 | 1100 | 07h30 | Kr+ga+Mg2SiO4+hiCapx |
| Ma91B | per | 14/8 | LaCr | 14·0 | 1100 | 09h25 | Kr+pX+ga+Mg2SiO4+hiCapx |
| Ma92B | per | 14/8 | LaCr | 15·0 | 1100 | 09h40 | pX+ga+Mg2SiO4+hiCapx+loCapx |
| Ma102M | per | 14/8 | LaCr | 18·0 | 1300 | 09h45 | pX+ga+Mg2SiO4+hiCapx+Q |
| Ma104M | per | 14/8 | LaCr | 13·0 | 1400 | 10h00 | ga+hiCapx+Mg2SiO4+Q |
| JKW7 | per | 10/5 | Re | 14·0 | 1300 | 07h00 | pX+ga+hiCapx+Mg2SiO4+loCapx+Q |
| JKW9 | per | 10/5 | Re | 12·0 | 1300 | 06h08 | Kr+pX+ga+hiCapx+Mg2SiO4+Q |
| JKW13 | per | 10/5 | Re | 10·0 | 1200 | 12h00 | Kr+pyr+phl+ga+hiCapx+Mg2SiO4+Q |
| JKW14 | per | 10/5 | Re | 14·0 | 1200 | 12h00 | Kr+pX+ga+Mg2SiO4+hiCapx+ |
| loCapx+Q | |||||||
| JKW15 | per | 10/5 | Re | 10·0 | 1300 | 48h00 | Kr+pyr+phl+ga+hiCapx+Mg2SiO4+Q |
| JKW16 | per | 8/3 | Re | 20·0 | 1300 | 07h00 | pX+ Mg2SiO4+ga+K-holl+Ca-perov |
| JKW17 | per | 10/5 | Re | 11·0 | 1300 | 48h00 | Kr+hiCapx+ga+Mg2SiO4+Q |
| JKW18 | per | 10/5 | Re | 10·0 | 1350 | 08h00 | Kr+phl+ga+hiCapx+Mg2SiO4+Q |
| JKW19 | per | 10/5 | Re | 10·0 | 1100 | 10h00 | Kr+phl+ga+hiCapx+Mg2SiO4+Q |
| JKW25 | per | 10/5 | Re | 15·0 | 900 | 36h00 | Kr+pX+pyr+hiCapx+loCapx+ga+ |
| Mg2SiO4 | |||||||
| Ma95sB | sub | 14/8 | LaCr | 10·0 | 1100 | 10h00 | Mg2SiO4+hiCapx+ga+Kr |
| Ma94sB | sub | 14/8 | LaCr | 15·0 | 1100 | 09h48 | Mg2SiO4+hiCapx+loCapx+ga+pX |
| JKW29 | sub | 10/5 | Re | 14·0 | 1100 | 11h16 | Mg2SiO4+hiCapx+ga+pX |
| JKW41 | sub | 10/5 | Re | 13·0 | 1300 | 11h45 | Mg2SiO4+hiCapx+loCapx+ga+Kr |
| JKW33 | sub | 10/5 | Re | 14·0 | 1400 | 12h00 | Mg2SiO4+hiCapx+loCapx+ga+pX |
| JKW34 | sub | 10/5 | Re | 14·0 | 900 | 48h00 | hiCapx+loCapx +ga+phase E+pX |
| JKW30 | sub | 10/5 | Re | 13·0 | 1100 | 30h00 | Mg2SiO4+hiCapx+loCapx+ga+Kr |
| JKW47 | sub | 8/3 | Re | 20·0 | 1500 | 05h00 | pX+Mg2SiO4+ga+K-holl+Ca-perov |
| JKW54 | sub | 8/3 | Re | 23·0 | 1700 | 03h00 | Mg2SiO4+ga+K-holl+Ca-perov+Q |
| JKW61 | sub | 8/3 | Re | 23·0 | 1500 | 12h00 | Mg2SiO4+ga+K-holl+Ca-perov+Q |
| JKW64 | sub | 8/3 | Re | 20·0 | 1600 | 06h00 | Mg2SiO4+ga+pX+Ca-perov+Q |
| JKW66 | sub | 8/3 | Re | 14·0 | 1600 | 08h00 | ga+loCapx+hiCapX+Q |
| JKW67 | sub | 8/3 | Re | 20·0 | 1800* | 03h00 | ga+Ca-perov+pX+Q |
| JKW63 | KLB | 10/5 | Re | 12·0 | 1200 | 72h00 | ga+loCapx+hiCapx+Mg2SiO4+Kr |
| JKW69 | KLB | 10/5 | Re | 14·0 | 1200 | 48h00 | ga+loCapx+hiCapX+Mg2SiO4+pX |
| JKW70 | KLB | 10/5 | Re | 13·0 | 1200 | 72h00 | ga+loCapx+hiCapX+ol |
Abbreviations of mineral phases are given in Table A1; per, peralkaline KNCMASH system; sub, subalkaline KNCMASH system; KLB = KLB-1 + 10 wt % synthetic K-richterite.
*Capsule slightly off-center, therefore actual T was probably not higher than 1700°C.
Sample capsules from completed experiments were embedded in epoxy resin and ground to expose the center of the charges. Most phase compositions were analyzed with an electron microprobe at analytical conditions of 15 kV and 20 nA. Phase X, which was found to be extremely susceptible to beam damage and loss of alkalis, was analyzed with 5 nA beam current and a rastered electron beam as large as the size of phase X grains permitted (typically 10–20 μm). Counting times of 20 s on peaks and 10 s on backgrounds of the X-ray lines were ratioed to a combination of synthetic oxide (Si, Mg, Al), synthetic mineral (Na) and natural mineral (Ca, K) standards. Data were corrected on-line using the PRZ correction procedure. After standardization, no peak search procedures were performed on phase X grains, to minimize residence time of the electron beam. Microprobe analyses of phlogopite and amphibole–pyribole were recalculated assuming stoichiometric OH. No recalculation was attempted for phase X because H2O was not determined quantitatively.
To search for structural OH in phase X, Raman spectra were recorded at the GL with a Dilor XY confocal micro Raman spectrometer equipped with a cryogenic Wright Model CCD. The excitation source was the 514 nm line of a Coherent Innova Model 90-5 Ar+ laser operating at 150 mW power using an integration time of 600 s.
PREVIOUS EXPERIMENTAL WORK
Phase X was described as a breakdown product of K-amphibole at P > 14 GPa between 1100 and 1400°C in the KCMSH system by Inoue et al. (1995a, 1998). Luth (1995, 1997) observed phase X in the system phlogopite–diopside at P ≥ 11 GPa. Based on secondary ionization mass spectrometry (SIMS) measurements of OH combined with microprobe analyses, Inoue et al. (1995a) proposed a formula of K4Mg8Si8O25(OH)2 for phase X. Experimental results of Luth (1997) and Inoue et al. (1998) show a wide range in K2O contents (10·2–19·4 wt % K2O) and oxide totals (91·5–98·2 wt %) at relatively constant Si:Mg or Si:(Mg + Al) ratios of 1:1. Phase X may be the ‘amphibole-like mineral’ reported by Trønnes (1990) as a breakdown product of phlogopite between 11 and 12 GPa. The formula given by Trønnes (1990)—K3·3Mg6·5Al0·5Si7O22(OH)2—is similar to the composition of phase X reported by Luth (1997) and Inoue et al. (1998).
RESULTS
Petrography and chemical homogeneity of the phases
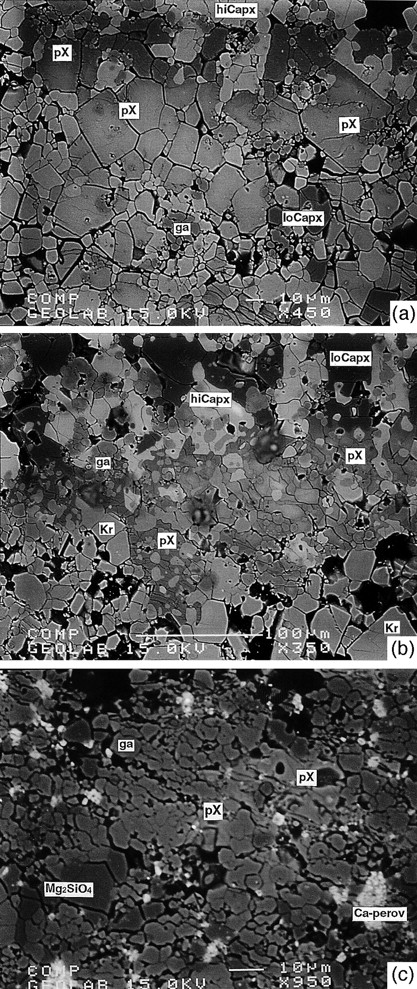
All starting materials readily recrystallize to mineral grains ∼10–50 μm in size at T ≥ 1100°C and to grains ≤10 μm in size in lower T runs (Fig. 1). HPPs are typically euhedral to subhedral but phase X also forms irregular grains that contain numerous olivine, clinopyroxene, or amphibole inclusions. Many phase X grains show irregularly spaced cleavage (Fig. 1b). A mixed-chain hydrous pyribole (sensuVeblen, 1981) was present in three runs, along with K-richterite. The former occurs as lath-shaped to needle-like crystals up to 100 μm × 20 μm in size. In high-P runs, K-hollandite appears as small (≤20 μm × 5 μm) needle-like crystals, and Ca-perovskite forms irregular patches up to 20 μm in size dispersed in the matrix or is present as inclusions in garnet. The phase distribution within individual capsules is inhomogeneous, and melt or quenched fluid is most abundant in the hotter part of the capsule. In experiments on the peralkaline bulk compositions, evidence for quench crystallization is present over a T interval of ≥200°C. The modal amounts of quench vary from <3% at 10 GPa and 1100°C to ∼20% at 10 GPa and 1350°C. Near the hot ends of the capsules, along the solid–liquid and quench interface, garnet and clinopyroxene often display larger grain sizes compared with cooler parts of the capsule. Inhomogeneities in grain size and phase distribution can be ascribed to grain maturation and chemical diffusion in a temperature gradient (Lesher & Walker, 1988) aided by the water content of the systems. With the exceptions of large garnets and phase X, neither systematic zoning of individual mineral grains nor differences in phase compositions are observed between tops and bottoms of the capsules. In low-T runs, diffuse Al-rich cores in garnets can be ascribed to incomplete equilibration. Phase X may show strong zoning with respect to K/(K + Na). This zoning is fairly regular, with K-rich cores and Na-rich rims, or is patchy and irregular. These compositional inhomogeneities are independent of run duration and temperature.
Back-scattered electron photomicrographs of phase X-bearing assemblages taken from the centers of experimental charges (tops of the images point to the hotter end of the capsules). (a) Run JKW7 at 14 GPa and 1300°C showing euhedral crystals of phase X in part with heterogeneity in K/(K + Na): light areas are K rich, whereas dark areas are more Na rich. (b) Run JKW9 at 12 GPa and 1300°C with coexisting phase X and K-richterite. Phase X shows irregular cleavage and numerous inclusions of Kr and hiCapx. Holes in the sample surface are due to mechanical abrasion during polishing. (c) Run JKW47 at 20 GPa and 1500°C showing poikiloblastic phase X coexisting with majoritic garnet and γ-Mg2SiO4; abbreviations are given in Table A1.
Phase relations
Peralkaline KNCMASH
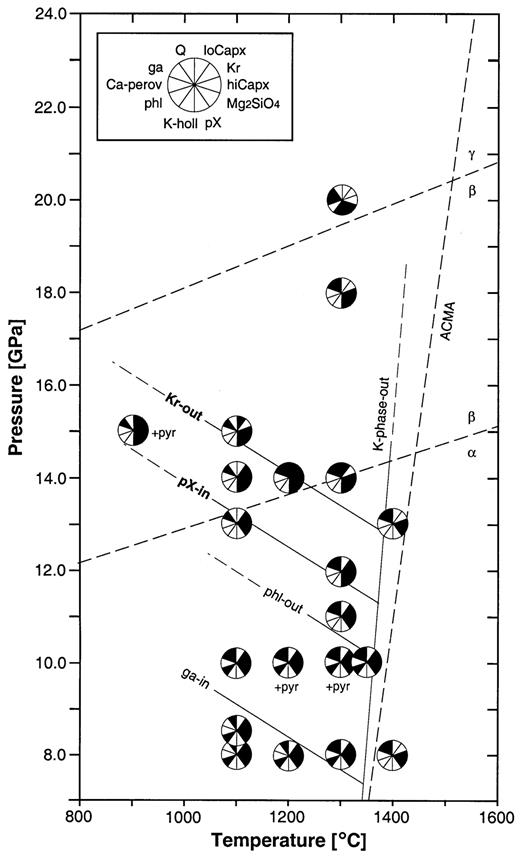
In the peralkaline KNCMASH system, HPPs are stable to at least 20 GPa and 1300°C, along with garnet + Mg2SiO4 + high-Ca clinopyroxene ± low-Ca clinopyroxene ± K-hollandite ± Ca-perovskite (Fig. 2). With increasing pressure, the first HPP to disappear is phlogopite, which is stable at 10 GPa between 1100°C and 1350°C along with K-richterite, but absent from run JKW17 at 11 GPa and 1300°C. In runs at 11 GPa and 1300°C, and 13 GPa and 1100°C K-richterite is the only stable HPP. At higher pressures, amphibole is joined by phase X as a result of continuous amphibole breakdown. At ≥15 GPa and 1100°C, and 14 GPa and 1300°C, the upper pressure stability limit of K-richterite is reached and amphibole is replaced by phase X as the HPP. Within the spacing of experimental data points, both phase X-in and K-richterite-out reactions have negative slopes with 5 MPa/K < dP/dT < 15 MPa/K for phase X-in and ≤10 MPa/K for K-richterite-out. At 20 GPa and 1300°C, K-hollandite appears as the first anhydrous potassic phase as a result of continuous phase X breakdown. Between 18 and 20 GPa, high-Ca clinopyroxene breaks down, and its diopside and jadeite components form Ca-perovskite and sodium-garnet solid solution, respectively.
P–T diagram of experimental results in the peralkaline KNCMASH system (Tables 1 and 2). Phases present in the experimental charges are represented by black sectors within the run symbol; phases not detected are denoted by white sectors (inset upper left); abbreviations are given in Table A1; ACMA average current mantle adiabat (see text); α- to β-Mg2SiO4 and β- to γ-Mg2SiO4 transitions according to Morishima et al. (1994) and Katsura & Ito (1989), respectively; experimental data at P < 10 GPa from Konzett et al. (1997) for comparison.
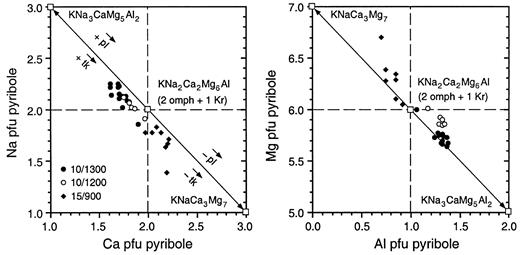
The high-temperature stability limit of all HPPs is reached between 1300 and 1400°C. At conditions of 8 GPa and 1400°C, and 13 GPa and 1400°C the stable assemblage is garnet + high-Ca clinopyroxene + Mg2SiO4 + quench (the term ‘quench’ is used to denote a mixture of unidentified and mostly K-rich phases that crystallized from a solute-rich fluid or a hydrous melt upon quenching). The spacing of experimental data points (Fig. 2) precludes discussion of the slope of the K-phase-out reaction(s), but in accordance with results at P ≤ 8·5 GPa (Konzett et al., 1997), we chose a positive slope. Because of the difficulty in distinguishing quenched melts from solute-rich fluids at high pressures based on textural evidence (see Konzett et al., 1997) we did not attempt to locate the position of the solidus. The mixed-chain hydrous pyribole was found in runs at 10 GPa and 15 GPa (Table 3), either with K-richterite + phlogopite or K-richterite alone (see Finger et al., 1998; Konzett & Fei, 1998).
Average analyses of K-richterite and mixed-chain hydrous pyribole
| Exp.: | JKW19 | JKW13 | JKW15 | JKW18 | JKW17 | JKW9 | Ma88sB | Ma91B | JKW14 | JKW25 | Ma95sB | JKW30 | JKW41 | |||
| Bulk: | per | per | per | per | per | per | per | per | per | per | sub | sub | sub | |||
| P (GPa): | 10 | 10 | 10 | 10 | 11 | 12 | 13 | 14 | 14 | 15 | 10 | 13 | 13 | |||
| T (°C): | 1100 | 1200 | 1300 | 1350 | 1300 | 1300 | 1100 | 1100 | 1200 | 900 | 1100 | 1100 | 1300 | |||
| amph | amph | pyr | amph | pyr | amph | amph | amph | amph | amph | amph | amph | pyr | amph | amph | amph | |
| No. of | ||||||||||||||||
| analyses: | 9 | 10 | 6 | 10 | 13 | 6 | 13 | 9 | 7 | 9 | 6 | 3 | 8 | 8 | 5 | 6 |
| SiO2 | 56·1(2) | 55·0(2) | 56·4(3) | 55·8(4) | 57·2(2) | 54·5(5) | 55·9(3) | 55·9(4) | 57·1(3) | 57·5(6) | 56·9(4) | 56·4(6) | 57·2(4) | 56·4(1) | 56·8(6) | 56·4(3) |
| Al2O3 | 2·8(1) | 2·9(2) | 5·2(3) | 2·5(1) | 5·2(3) | 3·5(1) | 3·0(2) | 1·7(2) | 0·8(1) | 0·6(0) | 0·5(1) | 0·3(3) | 3·3(3) | 1·0(1) | 0·5(5) | 0·7(0) |
| MgO | 23·7(1) | 23·0(2) | 18·8(2) | 23·1(2) | 18·4(3) | 22·8(3) | 23·0(3) | 23·1(3) | 24·2(2) | 24·0(1) | 23·7(1) | 23·7(3) | 20·2(8) | 24·2(1) | 23·9(4) | 23·5(2) |
| CaO | 7·1(2) | 6·7(4) | 8·2(3) | 6·6(2) | 7·6(4) | 7·0(2) | 6·2(4) | 6·1(3) | 6·3(2) | 6·2(1) | 6·4(2) | 6·3(3) | 9·5(4) | 6·8(1) | 6·2(1) | 5·8(1) |
| Na2O | 3·1(1) | 2·9(2) | 5·0(2) | 2·8(2) | 5·3(3) | 2·7(1) | 2·7(3) | 2·5(2) | 2·6(1) | 2·9(1) | 2·8(2) | 2·9(1) | 4·2(3) | 2·4(1) | 2·4(1) | 2·1(1) |
| K2O | 6·3(1) | 6·8(2) | 4·4(1) | 6·8(1) | 4·3(0) | 6·8(1) | 7·5(1) | 7·7(1) | 7·6(2) | 7·1(1) | 7·3(1) | 7·5(1) | 4·3(2) | 7·3(2) | 7·8(2) | 8·4(1) |
| H2O | 2·2(0) | 2·1(0) | 1·4(0) | 2·2(0) | 1·4(0) | 2·1(0) | 2·2(0) | 2·1(0) | 2·2(0) | 2·2(0) | 2·1(0) | 2·1(0) | 1·4(0) | 2·2(0) | 2·1(0) | 2·1(0) |
| ∑ | 101·2(5) | 99·3(3) | 99·4(4) | 99·8(6) | 99·4(3) | 99·4(7) | 100·5(3) | 99·1(5) | 100·7(5) | 100·7(7) | 99·6(4) | 99·4(1) | 100·2(8) | 100·2(2) | 99·8(8) | 99·1(5) |
| Si | 7·73(1) | 7·73(1) | 11·86(2) | 7·79(2) | 11·98(2) | 7·66(3) | 7·77(3) | 7·89(1) | 7·93(1) | 7·96(2) | 7·98(2) | 7·95(1) | 11·97(3) | 7·87(1) | 7·96(2) | 7·98(1) |
| Al | 0·45(1) | 0·48(3) | 1·29(6) | 0·41(1) | 1·29(8) | 0·58(2) | 0·49(4) | 0·29(3) | 0·13(1) | 0·12(1) | 0·08(1) | 0·05(1) | 0·80(7) | 0·17(1) | 0·10(1) | 0·12(0) |
| Mg | 4·86(1) | 4·81(4) | 5·90(6) | 4·81(3) | 5·74(9) | 4·79(1) | 4·77(6) | 4·85(4) | 5·00(3) | 4·95(0) | 4·95(4) | 4·98(2) | 6·30(2) | 5·03(2) | 5·00(6) | 4·94(2) |
| Ca | 1·05(2) | 1·01(5) | 1·84(7) | 0·99(3) | 1·71(8) | 1·06(3) | 0·93(6) | 0·92(4) | 0·93(1) | 0·92(1) | 0·96(3) | 0·96(4) | 2·13(9) | 1·01(1) | 0·92(2) | 0·88(1) |
| Na | 0·84(3) | 0·78(6) | 2·04(7) | 0·76(5) | 2·14(9) | 0·73(3) | 0·73(7) | 0·68(5) | 0·69(2) | 0·78(2) | 0·75(5) | 0·80(1) | 1·69(1) | 0·64(3) | 0·65(4) | 0·57(1) |
| K | 1·19(1) | 1·21(3) | 1·19(2) | 1·22(2) | 1·15(1) | 1·22(3) | 1·33(2) | 1·39(3) | 1·34(3) | 1·26(1) | 1·30(2) | 1·35(1) | 1·16(5) | 1·30(3) | 1·40(4) | 1·52(3) |
| ∑ | 16·02(2) | 16·02(1) | 24·11(1) | 15·99(2) | 24·01(4) | 16·02(2) | 16·02(1) | 16·01(3) | 16·02(1) | 16·01(1) | 16·01(3) | 16·10(1) | 24·05(3) | 16·02(1) | 16·02(4) | 16·01(2) |
| K/(K+Na) | 0·57(1) | 0·61(2) | 0·37(1) | 0·62(2) | 0·35(1) | 0·63(2) | 0·65(2) | 0·67(2) | 0·66(1) | 0·62(1) | 0·64(2) | 0·58(1) | 0·41(2) | 0·67(1) | 0·68(1) | 0·73(1) |
| Exp.: | JKW19 | JKW13 | JKW15 | JKW18 | JKW17 | JKW9 | Ma88sB | Ma91B | JKW14 | JKW25 | Ma95sB | JKW30 | JKW41 | |||
| Bulk: | per | per | per | per | per | per | per | per | per | per | sub | sub | sub | |||
| P (GPa): | 10 | 10 | 10 | 10 | 11 | 12 | 13 | 14 | 14 | 15 | 10 | 13 | 13 | |||
| T (°C): | 1100 | 1200 | 1300 | 1350 | 1300 | 1300 | 1100 | 1100 | 1200 | 900 | 1100 | 1100 | 1300 | |||
| amph | amph | pyr | amph | pyr | amph | amph | amph | amph | amph | amph | amph | pyr | amph | amph | amph | |
| No. of | ||||||||||||||||
| analyses: | 9 | 10 | 6 | 10 | 13 | 6 | 13 | 9 | 7 | 9 | 6 | 3 | 8 | 8 | 5 | 6 |
| SiO2 | 56·1(2) | 55·0(2) | 56·4(3) | 55·8(4) | 57·2(2) | 54·5(5) | 55·9(3) | 55·9(4) | 57·1(3) | 57·5(6) | 56·9(4) | 56·4(6) | 57·2(4) | 56·4(1) | 56·8(6) | 56·4(3) |
| Al2O3 | 2·8(1) | 2·9(2) | 5·2(3) | 2·5(1) | 5·2(3) | 3·5(1) | 3·0(2) | 1·7(2) | 0·8(1) | 0·6(0) | 0·5(1) | 0·3(3) | 3·3(3) | 1·0(1) | 0·5(5) | 0·7(0) |
| MgO | 23·7(1) | 23·0(2) | 18·8(2) | 23·1(2) | 18·4(3) | 22·8(3) | 23·0(3) | 23·1(3) | 24·2(2) | 24·0(1) | 23·7(1) | 23·7(3) | 20·2(8) | 24·2(1) | 23·9(4) | 23·5(2) |
| CaO | 7·1(2) | 6·7(4) | 8·2(3) | 6·6(2) | 7·6(4) | 7·0(2) | 6·2(4) | 6·1(3) | 6·3(2) | 6·2(1) | 6·4(2) | 6·3(3) | 9·5(4) | 6·8(1) | 6·2(1) | 5·8(1) |
| Na2O | 3·1(1) | 2·9(2) | 5·0(2) | 2·8(2) | 5·3(3) | 2·7(1) | 2·7(3) | 2·5(2) | 2·6(1) | 2·9(1) | 2·8(2) | 2·9(1) | 4·2(3) | 2·4(1) | 2·4(1) | 2·1(1) |
| K2O | 6·3(1) | 6·8(2) | 4·4(1) | 6·8(1) | 4·3(0) | 6·8(1) | 7·5(1) | 7·7(1) | 7·6(2) | 7·1(1) | 7·3(1) | 7·5(1) | 4·3(2) | 7·3(2) | 7·8(2) | 8·4(1) |
| H2O | 2·2(0) | 2·1(0) | 1·4(0) | 2·2(0) | 1·4(0) | 2·1(0) | 2·2(0) | 2·1(0) | 2·2(0) | 2·2(0) | 2·1(0) | 2·1(0) | 1·4(0) | 2·2(0) | 2·1(0) | 2·1(0) |
| ∑ | 101·2(5) | 99·3(3) | 99·4(4) | 99·8(6) | 99·4(3) | 99·4(7) | 100·5(3) | 99·1(5) | 100·7(5) | 100·7(7) | 99·6(4) | 99·4(1) | 100·2(8) | 100·2(2) | 99·8(8) | 99·1(5) |
| Si | 7·73(1) | 7·73(1) | 11·86(2) | 7·79(2) | 11·98(2) | 7·66(3) | 7·77(3) | 7·89(1) | 7·93(1) | 7·96(2) | 7·98(2) | 7·95(1) | 11·97(3) | 7·87(1) | 7·96(2) | 7·98(1) |
| Al | 0·45(1) | 0·48(3) | 1·29(6) | 0·41(1) | 1·29(8) | 0·58(2) | 0·49(4) | 0·29(3) | 0·13(1) | 0·12(1) | 0·08(1) | 0·05(1) | 0·80(7) | 0·17(1) | 0·10(1) | 0·12(0) |
| Mg | 4·86(1) | 4·81(4) | 5·90(6) | 4·81(3) | 5·74(9) | 4·79(1) | 4·77(6) | 4·85(4) | 5·00(3) | 4·95(0) | 4·95(4) | 4·98(2) | 6·30(2) | 5·03(2) | 5·00(6) | 4·94(2) |
| Ca | 1·05(2) | 1·01(5) | 1·84(7) | 0·99(3) | 1·71(8) | 1·06(3) | 0·93(6) | 0·92(4) | 0·93(1) | 0·92(1) | 0·96(3) | 0·96(4) | 2·13(9) | 1·01(1) | 0·92(2) | 0·88(1) |
| Na | 0·84(3) | 0·78(6) | 2·04(7) | 0·76(5) | 2·14(9) | 0·73(3) | 0·73(7) | 0·68(5) | 0·69(2) | 0·78(2) | 0·75(5) | 0·80(1) | 1·69(1) | 0·64(3) | 0·65(4) | 0·57(1) |
| K | 1·19(1) | 1·21(3) | 1·19(2) | 1·22(2) | 1·15(1) | 1·22(3) | 1·33(2) | 1·39(3) | 1·34(3) | 1·26(1) | 1·30(2) | 1·35(1) | 1·16(5) | 1·30(3) | 1·40(4) | 1·52(3) |
| ∑ | 16·02(2) | 16·02(1) | 24·11(1) | 15·99(2) | 24·01(4) | 16·02(2) | 16·02(1) | 16·01(3) | 16·02(1) | 16·01(1) | 16·01(3) | 16·10(1) | 24·05(3) | 16·02(1) | 16·02(4) | 16·01(2) |
| K/(K+Na) | 0·57(1) | 0·61(2) | 0·37(1) | 0·62(2) | 0·35(1) | 0·63(2) | 0·65(2) | 0·67(2) | 0·66(1) | 0·62(1) | 0·64(2) | 0·58(1) | 0·41(2) | 0·67(1) | 0·68(1) | 0·73(1) |
Amphibole formulae recalculated to 23 oxygens + stoichiometric OH; pyribole formulae recalculated to 33 oxygens + stoichiometric OH; numbers in parentheses denote 1σ SD.
Average analyses of K-richterite and mixed-chain hydrous pyribole
| Exp.: | JKW19 | JKW13 | JKW15 | JKW18 | JKW17 | JKW9 | Ma88sB | Ma91B | JKW14 | JKW25 | Ma95sB | JKW30 | JKW41 | |||
| Bulk: | per | per | per | per | per | per | per | per | per | per | sub | sub | sub | |||
| P (GPa): | 10 | 10 | 10 | 10 | 11 | 12 | 13 | 14 | 14 | 15 | 10 | 13 | 13 | |||
| T (°C): | 1100 | 1200 | 1300 | 1350 | 1300 | 1300 | 1100 | 1100 | 1200 | 900 | 1100 | 1100 | 1300 | |||
| amph | amph | pyr | amph | pyr | amph | amph | amph | amph | amph | amph | amph | pyr | amph | amph | amph | |
| No. of | ||||||||||||||||
| analyses: | 9 | 10 | 6 | 10 | 13 | 6 | 13 | 9 | 7 | 9 | 6 | 3 | 8 | 8 | 5 | 6 |
| SiO2 | 56·1(2) | 55·0(2) | 56·4(3) | 55·8(4) | 57·2(2) | 54·5(5) | 55·9(3) | 55·9(4) | 57·1(3) | 57·5(6) | 56·9(4) | 56·4(6) | 57·2(4) | 56·4(1) | 56·8(6) | 56·4(3) |
| Al2O3 | 2·8(1) | 2·9(2) | 5·2(3) | 2·5(1) | 5·2(3) | 3·5(1) | 3·0(2) | 1·7(2) | 0·8(1) | 0·6(0) | 0·5(1) | 0·3(3) | 3·3(3) | 1·0(1) | 0·5(5) | 0·7(0) |
| MgO | 23·7(1) | 23·0(2) | 18·8(2) | 23·1(2) | 18·4(3) | 22·8(3) | 23·0(3) | 23·1(3) | 24·2(2) | 24·0(1) | 23·7(1) | 23·7(3) | 20·2(8) | 24·2(1) | 23·9(4) | 23·5(2) |
| CaO | 7·1(2) | 6·7(4) | 8·2(3) | 6·6(2) | 7·6(4) | 7·0(2) | 6·2(4) | 6·1(3) | 6·3(2) | 6·2(1) | 6·4(2) | 6·3(3) | 9·5(4) | 6·8(1) | 6·2(1) | 5·8(1) |
| Na2O | 3·1(1) | 2·9(2) | 5·0(2) | 2·8(2) | 5·3(3) | 2·7(1) | 2·7(3) | 2·5(2) | 2·6(1) | 2·9(1) | 2·8(2) | 2·9(1) | 4·2(3) | 2·4(1) | 2·4(1) | 2·1(1) |
| K2O | 6·3(1) | 6·8(2) | 4·4(1) | 6·8(1) | 4·3(0) | 6·8(1) | 7·5(1) | 7·7(1) | 7·6(2) | 7·1(1) | 7·3(1) | 7·5(1) | 4·3(2) | 7·3(2) | 7·8(2) | 8·4(1) |
| H2O | 2·2(0) | 2·1(0) | 1·4(0) | 2·2(0) | 1·4(0) | 2·1(0) | 2·2(0) | 2·1(0) | 2·2(0) | 2·2(0) | 2·1(0) | 2·1(0) | 1·4(0) | 2·2(0) | 2·1(0) | 2·1(0) |
| ∑ | 101·2(5) | 99·3(3) | 99·4(4) | 99·8(6) | 99·4(3) | 99·4(7) | 100·5(3) | 99·1(5) | 100·7(5) | 100·7(7) | 99·6(4) | 99·4(1) | 100·2(8) | 100·2(2) | 99·8(8) | 99·1(5) |
| Si | 7·73(1) | 7·73(1) | 11·86(2) | 7·79(2) | 11·98(2) | 7·66(3) | 7·77(3) | 7·89(1) | 7·93(1) | 7·96(2) | 7·98(2) | 7·95(1) | 11·97(3) | 7·87(1) | 7·96(2) | 7·98(1) |
| Al | 0·45(1) | 0·48(3) | 1·29(6) | 0·41(1) | 1·29(8) | 0·58(2) | 0·49(4) | 0·29(3) | 0·13(1) | 0·12(1) | 0·08(1) | 0·05(1) | 0·80(7) | 0·17(1) | 0·10(1) | 0·12(0) |
| Mg | 4·86(1) | 4·81(4) | 5·90(6) | 4·81(3) | 5·74(9) | 4·79(1) | 4·77(6) | 4·85(4) | 5·00(3) | 4·95(0) | 4·95(4) | 4·98(2) | 6·30(2) | 5·03(2) | 5·00(6) | 4·94(2) |
| Ca | 1·05(2) | 1·01(5) | 1·84(7) | 0·99(3) | 1·71(8) | 1·06(3) | 0·93(6) | 0·92(4) | 0·93(1) | 0·92(1) | 0·96(3) | 0·96(4) | 2·13(9) | 1·01(1) | 0·92(2) | 0·88(1) |
| Na | 0·84(3) | 0·78(6) | 2·04(7) | 0·76(5) | 2·14(9) | 0·73(3) | 0·73(7) | 0·68(5) | 0·69(2) | 0·78(2) | 0·75(5) | 0·80(1) | 1·69(1) | 0·64(3) | 0·65(4) | 0·57(1) |
| K | 1·19(1) | 1·21(3) | 1·19(2) | 1·22(2) | 1·15(1) | 1·22(3) | 1·33(2) | 1·39(3) | 1·34(3) | 1·26(1) | 1·30(2) | 1·35(1) | 1·16(5) | 1·30(3) | 1·40(4) | 1·52(3) |
| ∑ | 16·02(2) | 16·02(1) | 24·11(1) | 15·99(2) | 24·01(4) | 16·02(2) | 16·02(1) | 16·01(3) | 16·02(1) | 16·01(1) | 16·01(3) | 16·10(1) | 24·05(3) | 16·02(1) | 16·02(4) | 16·01(2) |
| K/(K+Na) | 0·57(1) | 0·61(2) | 0·37(1) | 0·62(2) | 0·35(1) | 0·63(2) | 0·65(2) | 0·67(2) | 0·66(1) | 0·62(1) | 0·64(2) | 0·58(1) | 0·41(2) | 0·67(1) | 0·68(1) | 0·73(1) |
| Exp.: | JKW19 | JKW13 | JKW15 | JKW18 | JKW17 | JKW9 | Ma88sB | Ma91B | JKW14 | JKW25 | Ma95sB | JKW30 | JKW41 | |||
| Bulk: | per | per | per | per | per | per | per | per | per | per | sub | sub | sub | |||
| P (GPa): | 10 | 10 | 10 | 10 | 11 | 12 | 13 | 14 | 14 | 15 | 10 | 13 | 13 | |||
| T (°C): | 1100 | 1200 | 1300 | 1350 | 1300 | 1300 | 1100 | 1100 | 1200 | 900 | 1100 | 1100 | 1300 | |||
| amph | amph | pyr | amph | pyr | amph | amph | amph | amph | amph | amph | amph | pyr | amph | amph | amph | |
| No. of | ||||||||||||||||
| analyses: | 9 | 10 | 6 | 10 | 13 | 6 | 13 | 9 | 7 | 9 | 6 | 3 | 8 | 8 | 5 | 6 |
| SiO2 | 56·1(2) | 55·0(2) | 56·4(3) | 55·8(4) | 57·2(2) | 54·5(5) | 55·9(3) | 55·9(4) | 57·1(3) | 57·5(6) | 56·9(4) | 56·4(6) | 57·2(4) | 56·4(1) | 56·8(6) | 56·4(3) |
| Al2O3 | 2·8(1) | 2·9(2) | 5·2(3) | 2·5(1) | 5·2(3) | 3·5(1) | 3·0(2) | 1·7(2) | 0·8(1) | 0·6(0) | 0·5(1) | 0·3(3) | 3·3(3) | 1·0(1) | 0·5(5) | 0·7(0) |
| MgO | 23·7(1) | 23·0(2) | 18·8(2) | 23·1(2) | 18·4(3) | 22·8(3) | 23·0(3) | 23·1(3) | 24·2(2) | 24·0(1) | 23·7(1) | 23·7(3) | 20·2(8) | 24·2(1) | 23·9(4) | 23·5(2) |
| CaO | 7·1(2) | 6·7(4) | 8·2(3) | 6·6(2) | 7·6(4) | 7·0(2) | 6·2(4) | 6·1(3) | 6·3(2) | 6·2(1) | 6·4(2) | 6·3(3) | 9·5(4) | 6·8(1) | 6·2(1) | 5·8(1) |
| Na2O | 3·1(1) | 2·9(2) | 5·0(2) | 2·8(2) | 5·3(3) | 2·7(1) | 2·7(3) | 2·5(2) | 2·6(1) | 2·9(1) | 2·8(2) | 2·9(1) | 4·2(3) | 2·4(1) | 2·4(1) | 2·1(1) |
| K2O | 6·3(1) | 6·8(2) | 4·4(1) | 6·8(1) | 4·3(0) | 6·8(1) | 7·5(1) | 7·7(1) | 7·6(2) | 7·1(1) | 7·3(1) | 7·5(1) | 4·3(2) | 7·3(2) | 7·8(2) | 8·4(1) |
| H2O | 2·2(0) | 2·1(0) | 1·4(0) | 2·2(0) | 1·4(0) | 2·1(0) | 2·2(0) | 2·1(0) | 2·2(0) | 2·2(0) | 2·1(0) | 2·1(0) | 1·4(0) | 2·2(0) | 2·1(0) | 2·1(0) |
| ∑ | 101·2(5) | 99·3(3) | 99·4(4) | 99·8(6) | 99·4(3) | 99·4(7) | 100·5(3) | 99·1(5) | 100·7(5) | 100·7(7) | 99·6(4) | 99·4(1) | 100·2(8) | 100·2(2) | 99·8(8) | 99·1(5) |
| Si | 7·73(1) | 7·73(1) | 11·86(2) | 7·79(2) | 11·98(2) | 7·66(3) | 7·77(3) | 7·89(1) | 7·93(1) | 7·96(2) | 7·98(2) | 7·95(1) | 11·97(3) | 7·87(1) | 7·96(2) | 7·98(1) |
| Al | 0·45(1) | 0·48(3) | 1·29(6) | 0·41(1) | 1·29(8) | 0·58(2) | 0·49(4) | 0·29(3) | 0·13(1) | 0·12(1) | 0·08(1) | 0·05(1) | 0·80(7) | 0·17(1) | 0·10(1) | 0·12(0) |
| Mg | 4·86(1) | 4·81(4) | 5·90(6) | 4·81(3) | 5·74(9) | 4·79(1) | 4·77(6) | 4·85(4) | 5·00(3) | 4·95(0) | 4·95(4) | 4·98(2) | 6·30(2) | 5·03(2) | 5·00(6) | 4·94(2) |
| Ca | 1·05(2) | 1·01(5) | 1·84(7) | 0·99(3) | 1·71(8) | 1·06(3) | 0·93(6) | 0·92(4) | 0·93(1) | 0·92(1) | 0·96(3) | 0·96(4) | 2·13(9) | 1·01(1) | 0·92(2) | 0·88(1) |
| Na | 0·84(3) | 0·78(6) | 2·04(7) | 0·76(5) | 2·14(9) | 0·73(3) | 0·73(7) | 0·68(5) | 0·69(2) | 0·78(2) | 0·75(5) | 0·80(1) | 1·69(1) | 0·64(3) | 0·65(4) | 0·57(1) |
| K | 1·19(1) | 1·21(3) | 1·19(2) | 1·22(2) | 1·15(1) | 1·22(3) | 1·33(2) | 1·39(3) | 1·34(3) | 1·26(1) | 1·30(2) | 1·35(1) | 1·16(5) | 1·30(3) | 1·40(4) | 1·52(3) |
| ∑ | 16·02(2) | 16·02(1) | 24·11(1) | 15·99(2) | 24·01(4) | 16·02(2) | 16·02(1) | 16·01(3) | 16·02(1) | 16·01(1) | 16·01(3) | 16·10(1) | 24·05(3) | 16·02(1) | 16·02(4) | 16·01(2) |
| K/(K+Na) | 0·57(1) | 0·61(2) | 0·37(1) | 0·62(2) | 0·35(1) | 0·63(2) | 0·65(2) | 0·67(2) | 0·66(1) | 0·62(1) | 0·64(2) | 0·58(1) | 0·41(2) | 0·67(1) | 0·68(1) | 0·73(1) |
Amphibole formulae recalculated to 23 oxygens + stoichiometric OH; pyribole formulae recalculated to 33 oxygens + stoichiometric OH; numbers in parentheses denote 1σ SD.
Subalkaline KNCMASH and KLB-1
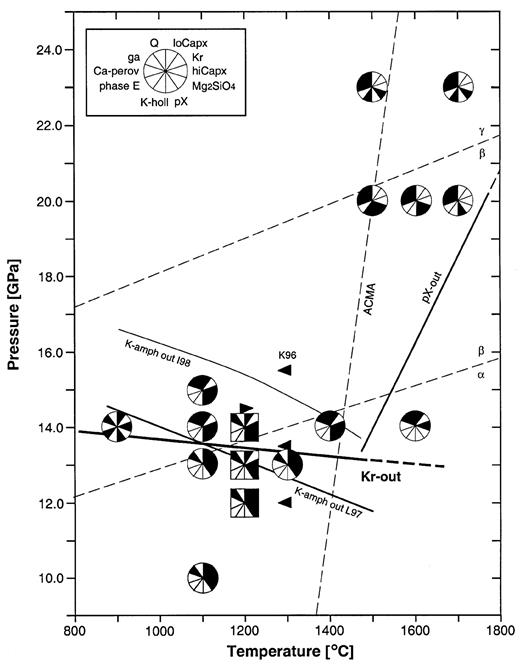
In the subalkaline system, K-richterite is stable to 13 GPa along with olivine + garnet + high-Ca clinopyroxene ± low-Ca clinopyroxene in an assemblage resembling a metasomatized lherzolite (Fig. 3). The apparent lack of low-Ca clinopyroxene in run Ma95sB is probably due to small grain sizes and the sparse occurrence of this phase in subalkaline runs. At P ≥ 14 GPa, K-richterite is replaced by phase X. The slope of the K-richterite-out reaction was assumed to be slightly negative, in accordance with the results of K-amphibole breakdown in the KCMSH system obtained by Inoue et al. (1998). Phase X is stable to at least 20 GPa. At this pressure, phase X may coexist with K-hollandite as a result of H2O partitioning constraints (see below), and with Ca-perovskite produced by the breakdown of high-Ca clinopyroxene. At 23 GPa, phase X is absent and K-hollandite carries the K in the system. The upper T stability limit of phase X is between 1400 and 1600°C at 14 GPa, in an assemblage without a solid K-rich phase: high-Ca clinopyroxene + garnet + low-Ca clinopyroxene + quench. At 20 GPa, the K-phase-out reaction must occur at T > 1700°C, which is at least 200°C above an average current mantle adiabat (ACMA) as defined by a β → γ transition in Mg2SiO4 at 17·9 GPa and 1475°C (Katsura & Ito, 1989) and a γ → perovskite + wüstite transition at 23·2 GPa and 1530°C (Ito & Takahashi, 1989).
P–T diagram of experimental results in the subalkaline KNCMASH (pie symbols) and the KLB-1 (square symbols) systems (Tables 1 and 2). Meaning of symbols, ACMA and α → β → γ transitions in Mg2SiO4 as in Fig. 2; K-amph out I98 and L97 indicate K-amphibole-out reactions in the KCMSH and KCMASH systems according to Inoue et al. (1998) and Luth (1997), respectively; filled arrows bracket position of H2O-saturated solidus for KLB-1 peridotite according to Kawamoto et al. (1996) (K96).
In the K-richterite-doped peridotite KLB-1, HPPs coexist with garnet + low-Ca clinopyroxene + high-Ca clinopyroxene + Mg2SiO4. K-richterite is stable at 12 GPa and 1200°C after a run duration of 72 h. X-ray mapping also showed that a K-rich phase is dispersed within graphite, along the interface between experimental charge and inner graphite capsule (see Konzett & Ulmer, 1999). At 13 GPa and 1200°C, after an identical run time, no HPP could be identified and X-ray mapping showed that all K was concentrated at the charge–graphite interface in diffuse grain boundary films. Because of their extremely small grain size, the K-carrying phase(s) could not be identified. Small amounts of alkaline and possibly CO2-rich fluid that probably formed by amphibole breakdown and/or graphite oxidation evidently destabilized phase X with increasing run durations. In a run that lasted only 48 h, phase X was stabilized at 14 GPa and 1200°C (see Table 9, below). Thus, the amphibole breakdown for starting material KLB-1 was placed between 12 and 13 GPa at 1200°C, which is 1 GPa below the amphibole → phase X transition in the subalkaline KNCMASH system (Fig. 3).
Representative analyses of coexisting phases from natural KLB-1 + 10% Kr
| Exp.: | JKW64 | JKW69 | ||||||||
| P (GPa): | 12·0 | 14·0 | ||||||||
| T (°C): | 1200 | 1200 | ||||||||
| ga | ol | hiCapx | loCapx | Kr | ga | β* | hiCapx | loCapx | pX | |
| SiO2 | 42·5 | 40·8 | 52·4 | 58·4 | 56·6 | 44·2 | 40·7 | 51·8 | 55·4 | 45·4 |
| TiO2 | 0·1 | <0·05 | 0·3 | <0·05 | 0·1 | 0·3 | <0·05 | 0·6 | 0·1 | 0·2 |
| Cr2O3 | 2·4 | <0·05 | 0·7 | <0·05 | 0·1 | 1·6 | 0·1 | 0·8 | 0·3 | 0·5 |
| Al2O3 | 21·2 | <0·05 | 6·8 | 0·1 | 0·8 | 18·6 | 0·1 | 7·1 | 3·7 | 0·5 |
| Fe2O3 | 1·9 | — | 2·0 | 1·1 | 1·9 | 4·4 | — | 2·5 | 2·4 | — |
| FeO | 7·8 | 9·3 | 1·5 | 4·3 | 0·6 | 3·1 | 10·9 | 1·0 | 2·2 | 6·7 |
| MnO | <0·05 | 0·1 | 0·1 | 0·1 | 0·1 | 0·5 | 0·1 | <0·05 | 0·2 | 0·1 |
| MgO | 20·8 | 50·3 | 15·2 | 36·3 | 22·7 | 23·1 | 49·1 | 15·2 | 34·1 | 28·3 |
| NiO | <0·05 | 0·4 | <0·05 | 0·2 | 0·1 | <0·05 | 0·4 | <0·05 | 0·1 | <0·05 |
| CaO | 3·9 | <0·05 | 19·8 | 0·2 | 5·8 | 4·9 | <0·05 | 20·0 | 1·4 | 0·3 |
| Na2O | 0·2 | <0·05 | 1·9 | 0·05 | 3·9 | 0·5 | 0·1 | 1·9 | 0·3 | 1·5 |
| K2O | <0·05 | <0·05 | <0·05 | <0·05 | 6·0 | <0·05 | <0·05 | 0·1 | <0·05 | 14·5 |
| H2O | — | — | — | — | 2·2 | — | — | — | — | |
| ∑ | 101·0 | 100·8 | 100·6 | 100·7 | 100·9 | 101·2 | 101·4 | 100·9 | 100·2 | 97·9 |
| Si | 3·01 | 0·99 | 1·88 | 1·99 | 7·89 | 3·09 | 0·99 | 1·86 | 1·90 | |
| Ti | 0·01 | — | 0·01 | — | 0·01 | 0·02 | — | 0·02 | 0·00 | |
| Cr | 0·13 | — | 0·02 | — | 0·01 | 0·09 | 0·00 | 0·02 | 0·01 | |
| Al | 1·77 | — | 0·29 | 0·00 | 0·13 | 1·54 | 0·00 | 0·30 | 0·15 | |
| Fe3+ | 0·10 | — | 0·06 | 0·03 | 0·20 | 0·23 | — | 0·07 | 0·06 | |
| Fe2+ | 0·46 | 0·19 | 0·04 | 0·12 | 0·07 | 0·18 | 0·22 | 0·03 | 0·06 | |
| Mn | 0·02 | 0·00 | 0·00 | 0·00 | 0·01 | 0·03 | 0·00 | — | 0·01 | |
| Mg | 2·19 | 1·82 | 0·81 | 1·84 | 4·71 | 2·40 | 1·77 | 0·81 | 1·74 | |
| Ni | — | 0·01 | — | 0·01 | 0·02 | — | 0·01 | — | 0·00 | |
| Ca | 0·30 | — | 0·76 | 0·01 | 0·87 | 0·37 | — | 0·77 | 0·05 | |
| Na | 0·02 | — | 0·13 | 0·00 | 1·04 | 0·06 | 0·00 | 0·13 | 0·02 | |
| K | — | — | — | — | 1·07 | — | — | 0·00 | — | |
| XMg(Fetot) | 0·80 | 0·90 | 0·90 | 0·93 | 0·95 | 0·85 | 0·89 | 0·89 | 0·93 | 0·88 |
| K/(K+Na) | — | — | 0·02 | — | 0·51 | — | — | 0·03 | — | 0·87 |
| Exp.: | JKW64 | JKW69 | ||||||||
| P (GPa): | 12·0 | 14·0 | ||||||||
| T (°C): | 1200 | 1200 | ||||||||
| ga | ol | hiCapx | loCapx | Kr | ga | β* | hiCapx | loCapx | pX | |
| SiO2 | 42·5 | 40·8 | 52·4 | 58·4 | 56·6 | 44·2 | 40·7 | 51·8 | 55·4 | 45·4 |
| TiO2 | 0·1 | <0·05 | 0·3 | <0·05 | 0·1 | 0·3 | <0·05 | 0·6 | 0·1 | 0·2 |
| Cr2O3 | 2·4 | <0·05 | 0·7 | <0·05 | 0·1 | 1·6 | 0·1 | 0·8 | 0·3 | 0·5 |
| Al2O3 | 21·2 | <0·05 | 6·8 | 0·1 | 0·8 | 18·6 | 0·1 | 7·1 | 3·7 | 0·5 |
| Fe2O3 | 1·9 | — | 2·0 | 1·1 | 1·9 | 4·4 | — | 2·5 | 2·4 | — |
| FeO | 7·8 | 9·3 | 1·5 | 4·3 | 0·6 | 3·1 | 10·9 | 1·0 | 2·2 | 6·7 |
| MnO | <0·05 | 0·1 | 0·1 | 0·1 | 0·1 | 0·5 | 0·1 | <0·05 | 0·2 | 0·1 |
| MgO | 20·8 | 50·3 | 15·2 | 36·3 | 22·7 | 23·1 | 49·1 | 15·2 | 34·1 | 28·3 |
| NiO | <0·05 | 0·4 | <0·05 | 0·2 | 0·1 | <0·05 | 0·4 | <0·05 | 0·1 | <0·05 |
| CaO | 3·9 | <0·05 | 19·8 | 0·2 | 5·8 | 4·9 | <0·05 | 20·0 | 1·4 | 0·3 |
| Na2O | 0·2 | <0·05 | 1·9 | 0·05 | 3·9 | 0·5 | 0·1 | 1·9 | 0·3 | 1·5 |
| K2O | <0·05 | <0·05 | <0·05 | <0·05 | 6·0 | <0·05 | <0·05 | 0·1 | <0·05 | 14·5 |
| H2O | — | — | — | — | 2·2 | — | — | — | — | |
| ∑ | 101·0 | 100·8 | 100·6 | 100·7 | 100·9 | 101·2 | 101·4 | 100·9 | 100·2 | 97·9 |
| Si | 3·01 | 0·99 | 1·88 | 1·99 | 7·89 | 3·09 | 0·99 | 1·86 | 1·90 | |
| Ti | 0·01 | — | 0·01 | — | 0·01 | 0·02 | — | 0·02 | 0·00 | |
| Cr | 0·13 | — | 0·02 | — | 0·01 | 0·09 | 0·00 | 0·02 | 0·01 | |
| Al | 1·77 | — | 0·29 | 0·00 | 0·13 | 1·54 | 0·00 | 0·30 | 0·15 | |
| Fe3+ | 0·10 | — | 0·06 | 0·03 | 0·20 | 0·23 | — | 0·07 | 0·06 | |
| Fe2+ | 0·46 | 0·19 | 0·04 | 0·12 | 0·07 | 0·18 | 0·22 | 0·03 | 0·06 | |
| Mn | 0·02 | 0·00 | 0·00 | 0·00 | 0·01 | 0·03 | 0·00 | — | 0·01 | |
| Mg | 2·19 | 1·82 | 0·81 | 1·84 | 4·71 | 2·40 | 1·77 | 0·81 | 1·74 | |
| Ni | — | 0·01 | — | 0·01 | 0·02 | — | 0·01 | — | 0·00 | |
| Ca | 0·30 | — | 0·76 | 0·01 | 0·87 | 0·37 | — | 0·77 | 0·05 | |
| Na | 0·02 | — | 0·13 | 0·00 | 1·04 | 0·06 | 0·00 | 0·13 | 0·02 | |
| K | — | — | — | — | 1·07 | — | — | 0·00 | — | |
| XMg(Fetot) | 0·80 | 0·90 | 0·90 | 0·93 | 0·95 | 0·85 | 0·89 | 0·89 | 0·93 | 0·88 |
| K/(K+Na) | — | — | 0·02 | — | 0·51 | — | — | 0·03 | — | 0·87 |
Mineral formulae recalculated as follows: α/β-Mg2SiO4 3 cat/4 ox; pyroxenes 4 cat/6 ox; ga 8 cat/12 ox; Kr 16 cat/23 ox + 2 OH.
*Verified by Raman spectroscopy.
Representative analyses of coexisting phases from natural KLB-1 + 10% Kr
| Exp.: | JKW64 | JKW69 | ||||||||
| P (GPa): | 12·0 | 14·0 | ||||||||
| T (°C): | 1200 | 1200 | ||||||||
| ga | ol | hiCapx | loCapx | Kr | ga | β* | hiCapx | loCapx | pX | |
| SiO2 | 42·5 | 40·8 | 52·4 | 58·4 | 56·6 | 44·2 | 40·7 | 51·8 | 55·4 | 45·4 |
| TiO2 | 0·1 | <0·05 | 0·3 | <0·05 | 0·1 | 0·3 | <0·05 | 0·6 | 0·1 | 0·2 |
| Cr2O3 | 2·4 | <0·05 | 0·7 | <0·05 | 0·1 | 1·6 | 0·1 | 0·8 | 0·3 | 0·5 |
| Al2O3 | 21·2 | <0·05 | 6·8 | 0·1 | 0·8 | 18·6 | 0·1 | 7·1 | 3·7 | 0·5 |
| Fe2O3 | 1·9 | — | 2·0 | 1·1 | 1·9 | 4·4 | — | 2·5 | 2·4 | — |
| FeO | 7·8 | 9·3 | 1·5 | 4·3 | 0·6 | 3·1 | 10·9 | 1·0 | 2·2 | 6·7 |
| MnO | <0·05 | 0·1 | 0·1 | 0·1 | 0·1 | 0·5 | 0·1 | <0·05 | 0·2 | 0·1 |
| MgO | 20·8 | 50·3 | 15·2 | 36·3 | 22·7 | 23·1 | 49·1 | 15·2 | 34·1 | 28·3 |
| NiO | <0·05 | 0·4 | <0·05 | 0·2 | 0·1 | <0·05 | 0·4 | <0·05 | 0·1 | <0·05 |
| CaO | 3·9 | <0·05 | 19·8 | 0·2 | 5·8 | 4·9 | <0·05 | 20·0 | 1·4 | 0·3 |
| Na2O | 0·2 | <0·05 | 1·9 | 0·05 | 3·9 | 0·5 | 0·1 | 1·9 | 0·3 | 1·5 |
| K2O | <0·05 | <0·05 | <0·05 | <0·05 | 6·0 | <0·05 | <0·05 | 0·1 | <0·05 | 14·5 |
| H2O | — | — | — | — | 2·2 | — | — | — | — | |
| ∑ | 101·0 | 100·8 | 100·6 | 100·7 | 100·9 | 101·2 | 101·4 | 100·9 | 100·2 | 97·9 |
| Si | 3·01 | 0·99 | 1·88 | 1·99 | 7·89 | 3·09 | 0·99 | 1·86 | 1·90 | |
| Ti | 0·01 | — | 0·01 | — | 0·01 | 0·02 | — | 0·02 | 0·00 | |
| Cr | 0·13 | — | 0·02 | — | 0·01 | 0·09 | 0·00 | 0·02 | 0·01 | |
| Al | 1·77 | — | 0·29 | 0·00 | 0·13 | 1·54 | 0·00 | 0·30 | 0·15 | |
| Fe3+ | 0·10 | — | 0·06 | 0·03 | 0·20 | 0·23 | — | 0·07 | 0·06 | |
| Fe2+ | 0·46 | 0·19 | 0·04 | 0·12 | 0·07 | 0·18 | 0·22 | 0·03 | 0·06 | |
| Mn | 0·02 | 0·00 | 0·00 | 0·00 | 0·01 | 0·03 | 0·00 | — | 0·01 | |
| Mg | 2·19 | 1·82 | 0·81 | 1·84 | 4·71 | 2·40 | 1·77 | 0·81 | 1·74 | |
| Ni | — | 0·01 | — | 0·01 | 0·02 | — | 0·01 | — | 0·00 | |
| Ca | 0·30 | — | 0·76 | 0·01 | 0·87 | 0·37 | — | 0·77 | 0·05 | |
| Na | 0·02 | — | 0·13 | 0·00 | 1·04 | 0·06 | 0·00 | 0·13 | 0·02 | |
| K | — | — | — | — | 1·07 | — | — | 0·00 | — | |
| XMg(Fetot) | 0·80 | 0·90 | 0·90 | 0·93 | 0·95 | 0·85 | 0·89 | 0·89 | 0·93 | 0·88 |
| K/(K+Na) | — | — | 0·02 | — | 0·51 | — | — | 0·03 | — | 0·87 |
| Exp.: | JKW64 | JKW69 | ||||||||
| P (GPa): | 12·0 | 14·0 | ||||||||
| T (°C): | 1200 | 1200 | ||||||||
| ga | ol | hiCapx | loCapx | Kr | ga | β* | hiCapx | loCapx | pX | |
| SiO2 | 42·5 | 40·8 | 52·4 | 58·4 | 56·6 | 44·2 | 40·7 | 51·8 | 55·4 | 45·4 |
| TiO2 | 0·1 | <0·05 | 0·3 | <0·05 | 0·1 | 0·3 | <0·05 | 0·6 | 0·1 | 0·2 |
| Cr2O3 | 2·4 | <0·05 | 0·7 | <0·05 | 0·1 | 1·6 | 0·1 | 0·8 | 0·3 | 0·5 |
| Al2O3 | 21·2 | <0·05 | 6·8 | 0·1 | 0·8 | 18·6 | 0·1 | 7·1 | 3·7 | 0·5 |
| Fe2O3 | 1·9 | — | 2·0 | 1·1 | 1·9 | 4·4 | — | 2·5 | 2·4 | — |
| FeO | 7·8 | 9·3 | 1·5 | 4·3 | 0·6 | 3·1 | 10·9 | 1·0 | 2·2 | 6·7 |
| MnO | <0·05 | 0·1 | 0·1 | 0·1 | 0·1 | 0·5 | 0·1 | <0·05 | 0·2 | 0·1 |
| MgO | 20·8 | 50·3 | 15·2 | 36·3 | 22·7 | 23·1 | 49·1 | 15·2 | 34·1 | 28·3 |
| NiO | <0·05 | 0·4 | <0·05 | 0·2 | 0·1 | <0·05 | 0·4 | <0·05 | 0·1 | <0·05 |
| CaO | 3·9 | <0·05 | 19·8 | 0·2 | 5·8 | 4·9 | <0·05 | 20·0 | 1·4 | 0·3 |
| Na2O | 0·2 | <0·05 | 1·9 | 0·05 | 3·9 | 0·5 | 0·1 | 1·9 | 0·3 | 1·5 |
| K2O | <0·05 | <0·05 | <0·05 | <0·05 | 6·0 | <0·05 | <0·05 | 0·1 | <0·05 | 14·5 |
| H2O | — | — | — | — | 2·2 | — | — | — | — | |
| ∑ | 101·0 | 100·8 | 100·6 | 100·7 | 100·9 | 101·2 | 101·4 | 100·9 | 100·2 | 97·9 |
| Si | 3·01 | 0·99 | 1·88 | 1·99 | 7·89 | 3·09 | 0·99 | 1·86 | 1·90 | |
| Ti | 0·01 | — | 0·01 | — | 0·01 | 0·02 | — | 0·02 | 0·00 | |
| Cr | 0·13 | — | 0·02 | — | 0·01 | 0·09 | 0·00 | 0·02 | 0·01 | |
| Al | 1·77 | — | 0·29 | 0·00 | 0·13 | 1·54 | 0·00 | 0·30 | 0·15 | |
| Fe3+ | 0·10 | — | 0·06 | 0·03 | 0·20 | 0·23 | — | 0·07 | 0·06 | |
| Fe2+ | 0·46 | 0·19 | 0·04 | 0·12 | 0·07 | 0·18 | 0·22 | 0·03 | 0·06 | |
| Mn | 0·02 | 0·00 | 0·00 | 0·00 | 0·01 | 0·03 | 0·00 | — | 0·01 | |
| Mg | 2·19 | 1·82 | 0·81 | 1·84 | 4·71 | 2·40 | 1·77 | 0·81 | 1·74 | |
| Ni | — | 0·01 | — | 0·01 | 0·02 | — | 0·01 | — | 0·00 | |
| Ca | 0·30 | — | 0·76 | 0·01 | 0·87 | 0·37 | — | 0·77 | 0·05 | |
| Na | 0·02 | — | 0·13 | 0·00 | 1·04 | 0·06 | 0·00 | 0·13 | 0·02 | |
| K | — | — | — | — | 1·07 | — | — | 0·00 | — | |
| XMg(Fetot) | 0·80 | 0·90 | 0·90 | 0·93 | 0·95 | 0·85 | 0·89 | 0·89 | 0·93 | 0·88 |
| K/(K+Na) | — | — | 0·02 | — | 0·51 | — | — | 0·03 | — | 0·87 |
Mineral formulae recalculated as follows: α/β-Mg2SiO4 3 cat/4 ox; pyroxenes 4 cat/6 ox; ga 8 cat/12 ox; Kr 16 cat/23 ox + 2 OH.
*Verified by Raman spectroscopy.
Mineral chemistry
K-amphibole and hydrous pyribole
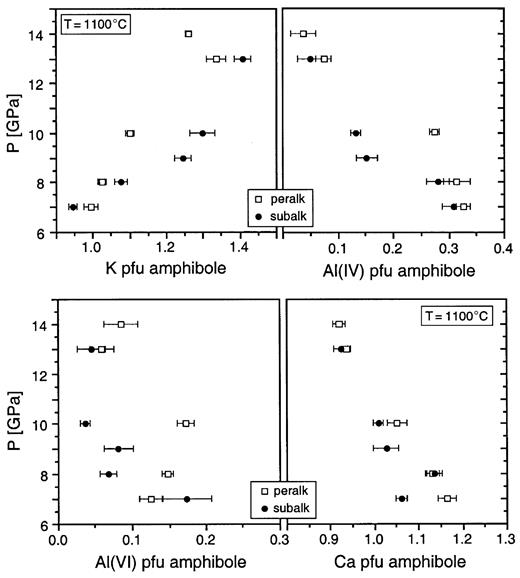
Selected mineral chemical parameters of K-richterite as a function of P at constant T (1100°C) for peralkaline and subalkaline KNCMASH systems; each point represents an average of 5–13 individual amphibole analyses (Table 3).
In the KLB-1 starting composition, amphibole at its upper P stability limit is still close to K-richterite end-member composition (see Table 9, below) with 1·07–1·13 K p.f.u. and 0·11–0·13 Al p.f.u. The XMg for amphibole is 0·95 with XMgKr > XMgloCapx > XMgol > XMghiCapx > XMgga.
Compositional variation of mixed-chain hydrous pyribole in terms of Na–Ca and Mg–Al; □, hypothetical pyribole end-member compositions (see text for explanation).
Phlogopite
Phlogopite is near the end-member composition, with a small excess of 0·02–0·04 Si p.f.u. (Table 4), indicating limited solid-solution with K(Mg2·5□0·5)Si4O10(OH)2 (Seifert & Schreyer, 1971).
Average analyses of phlogopite
| Exp.: | JKW19 | JKW13 | JKW15 | JKW18 |
| P (GPa): | 10 | 10 | 10 | 10 |
| T (°C): | 1100 | 1200 | 1300 | 1350 |
| No. of analyses: | 8 | 5 | 10 | 7 |
| SiO2 | 43·9(2) | 43·6(1) | 43·3(4) | 43·4(4) |
| Al2O3 | 11·8(1) | 11·5(1) | 11·8(2) | 11·6(2) |
| MgO | 28·9(2) | 28·5(2) | 28·7(1) | 28·2(2) |
| CaO | <0·05 | <0·05 | <0·05 | <0·05 |
| Na2O | 0·1(1) | 0·1(0) | 0·1(0) | 0·2(0) |
| K2O | 11·4(1) | 11·4(1) | 11·2(1) | 11·4(2) |
| H2O | 4·3(0) | 4·3(0) | 4·3(0) | 4·3(0) |
| ∑ | 100·4(5) | 99·5(4) | 99·4(6) | 99·0(7) |
| Si | 3·04(1) | 3·04(1) | 3·02(1) | 3·04(2) |
| Al | 0·96(0) | 0·95(1) | 0·97(1) | 0·96(1) |
| Mg | 2·98(2) | 2·97(1) | 2·99(2) | 2·95(2) |
| Ca | — | — | — | — |
| Na | 0·01(1) | 0·01(0) | 0·01(0) | 0·02(1) |
| K | 1·00(1) | 1·00(1) | 1·00(1) | 1·02(2) |
| ∑ | 7·99(1) | 8·00(1) | 7·99(1) | 7·99(1) |
| K/(K+Na) | 0·99(1) | 0·99(0) | 0·99(0) | 0·98(0) |
| Exp.: | JKW19 | JKW13 | JKW15 | JKW18 |
| P (GPa): | 10 | 10 | 10 | 10 |
| T (°C): | 1100 | 1200 | 1300 | 1350 |
| No. of analyses: | 8 | 5 | 10 | 7 |
| SiO2 | 43·9(2) | 43·6(1) | 43·3(4) | 43·4(4) |
| Al2O3 | 11·8(1) | 11·5(1) | 11·8(2) | 11·6(2) |
| MgO | 28·9(2) | 28·5(2) | 28·7(1) | 28·2(2) |
| CaO | <0·05 | <0·05 | <0·05 | <0·05 |
| Na2O | 0·1(1) | 0·1(0) | 0·1(0) | 0·2(0) |
| K2O | 11·4(1) | 11·4(1) | 11·2(1) | 11·4(2) |
| H2O | 4·3(0) | 4·3(0) | 4·3(0) | 4·3(0) |
| ∑ | 100·4(5) | 99·5(4) | 99·4(6) | 99·0(7) |
| Si | 3·04(1) | 3·04(1) | 3·02(1) | 3·04(2) |
| Al | 0·96(0) | 0·95(1) | 0·97(1) | 0·96(1) |
| Mg | 2·98(2) | 2·97(1) | 2·99(2) | 2·95(2) |
| Ca | — | — | — | — |
| Na | 0·01(1) | 0·01(0) | 0·01(0) | 0·02(1) |
| K | 1·00(1) | 1·00(1) | 1·00(1) | 1·02(2) |
| ∑ | 7·99(1) | 8·00(1) | 7·99(1) | 7·99(1) |
| K/(K+Na) | 0·99(1) | 0·99(0) | 0·99(0) | 0·98(0) |
Phlogopite formulae recalculated to 11 oxygens + stoichiometric OH.
Average analyses of phlogopite
| Exp.: | JKW19 | JKW13 | JKW15 | JKW18 |
| P (GPa): | 10 | 10 | 10 | 10 |
| T (°C): | 1100 | 1200 | 1300 | 1350 |
| No. of analyses: | 8 | 5 | 10 | 7 |
| SiO2 | 43·9(2) | 43·6(1) | 43·3(4) | 43·4(4) |
| Al2O3 | 11·8(1) | 11·5(1) | 11·8(2) | 11·6(2) |
| MgO | 28·9(2) | 28·5(2) | 28·7(1) | 28·2(2) |
| CaO | <0·05 | <0·05 | <0·05 | <0·05 |
| Na2O | 0·1(1) | 0·1(0) | 0·1(0) | 0·2(0) |
| K2O | 11·4(1) | 11·4(1) | 11·2(1) | 11·4(2) |
| H2O | 4·3(0) | 4·3(0) | 4·3(0) | 4·3(0) |
| ∑ | 100·4(5) | 99·5(4) | 99·4(6) | 99·0(7) |
| Si | 3·04(1) | 3·04(1) | 3·02(1) | 3·04(2) |
| Al | 0·96(0) | 0·95(1) | 0·97(1) | 0·96(1) |
| Mg | 2·98(2) | 2·97(1) | 2·99(2) | 2·95(2) |
| Ca | — | — | — | — |
| Na | 0·01(1) | 0·01(0) | 0·01(0) | 0·02(1) |
| K | 1·00(1) | 1·00(1) | 1·00(1) | 1·02(2) |
| ∑ | 7·99(1) | 8·00(1) | 7·99(1) | 7·99(1) |
| K/(K+Na) | 0·99(1) | 0·99(0) | 0·99(0) | 0·98(0) |
| Exp.: | JKW19 | JKW13 | JKW15 | JKW18 |
| P (GPa): | 10 | 10 | 10 | 10 |
| T (°C): | 1100 | 1200 | 1300 | 1350 |
| No. of analyses: | 8 | 5 | 10 | 7 |
| SiO2 | 43·9(2) | 43·6(1) | 43·3(4) | 43·4(4) |
| Al2O3 | 11·8(1) | 11·5(1) | 11·8(2) | 11·6(2) |
| MgO | 28·9(2) | 28·5(2) | 28·7(1) | 28·2(2) |
| CaO | <0·05 | <0·05 | <0·05 | <0·05 |
| Na2O | 0·1(1) | 0·1(0) | 0·1(0) | 0·2(0) |
| K2O | 11·4(1) | 11·4(1) | 11·2(1) | 11·4(2) |
| H2O | 4·3(0) | 4·3(0) | 4·3(0) | 4·3(0) |
| ∑ | 100·4(5) | 99·5(4) | 99·4(6) | 99·0(7) |
| Si | 3·04(1) | 3·04(1) | 3·02(1) | 3·04(2) |
| Al | 0·96(0) | 0·95(1) | 0·97(1) | 0·96(1) |
| Mg | 2·98(2) | 2·97(1) | 2·99(2) | 2·95(2) |
| Ca | — | — | — | — |
| Na | 0·01(1) | 0·01(0) | 0·01(0) | 0·02(1) |
| K | 1·00(1) | 1·00(1) | 1·00(1) | 1·02(2) |
| ∑ | 7·99(1) | 8·00(1) | 7·99(1) | 7·99(1) |
| K/(K+Na) | 0·99(1) | 0·99(0) | 0·99(0) | 0·98(0) |
Phlogopite formulae recalculated to 11 oxygens + stoichiometric OH.
Phase X
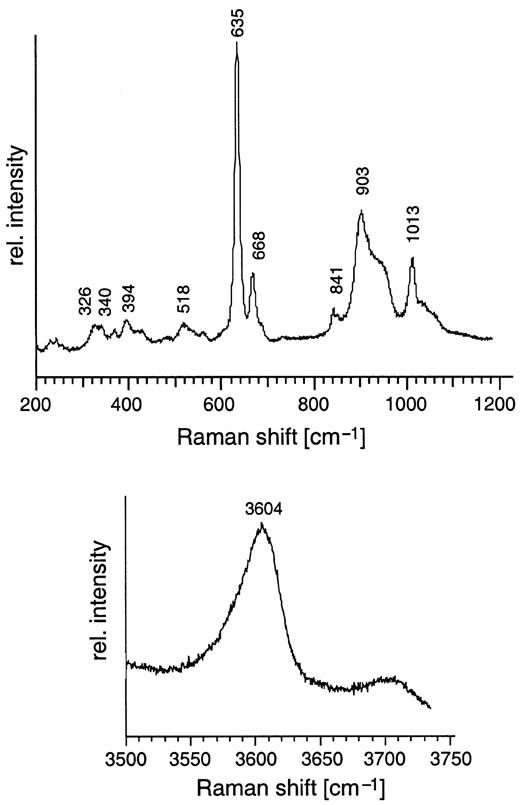
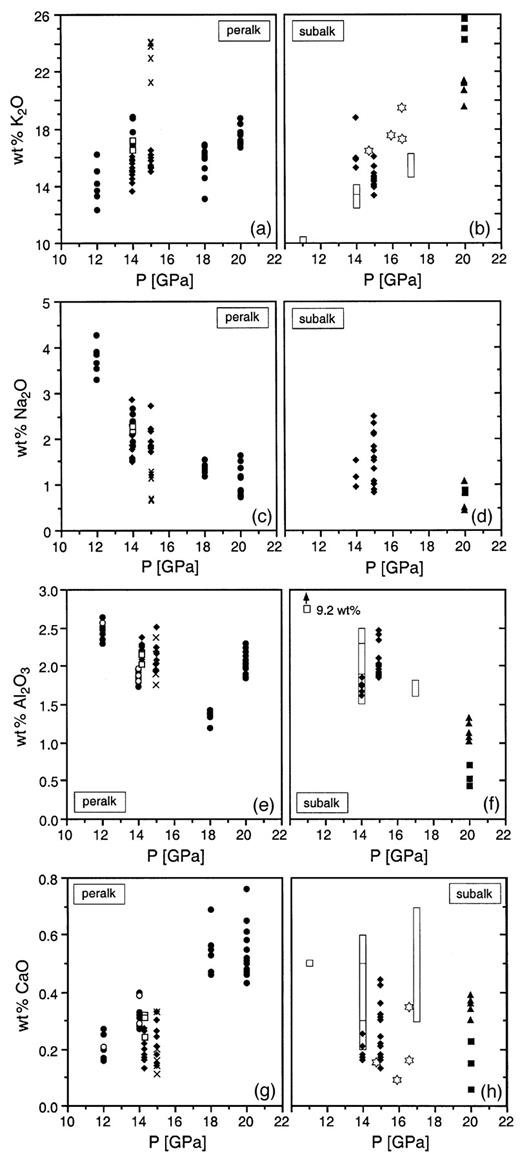
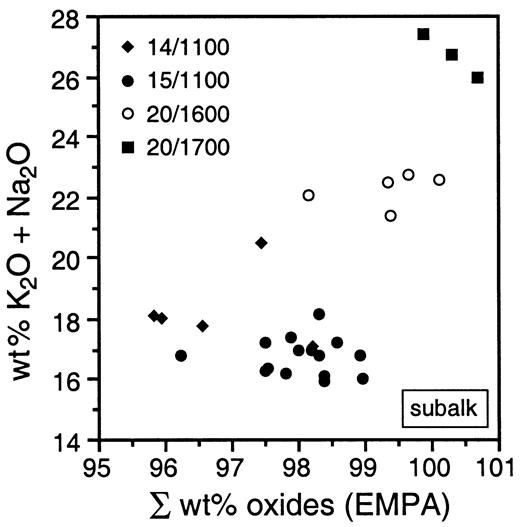
Phase X is a disilicate (Libau, 1982), containing corner-sharing SiO4 tetrahedra and has a stoichiometry A2–xB2Si2O7Hx with A = K or Na, B = Mg, Al or Ca, and x = 0–1, ranging from anhydrous A2B2Si2O7 to A□B2Si2O7H with a maximum possible H2O content of 3·51 wt % and a vacancy on the A-position (H. Yang & J. Konzett, unpublished data, 1999; Konzett & Yang, 1998). Variable H2O contents have been observed in phase E (Kudoh et al., 1993), phase D (Frost & Fei, 1998), hydrous wadsleyite (Kudoh et al., 1996) or hydrous modified spinel (Inoue et al., 1995b). The presence of OH in phase X in both subalkaline and peralkaline systems was confirmed by laser Raman spectroscopy. Raman spectra show strong peaks in the OH-stretching region at 3600 cm−1 (Fig. 6). This is consistent with low average analytical oxide totals in the range of 96–98 wt % in most runs (Table 5). In the silicate stretching region, phase X spectra are characterized by major peaks at 635–640 cm−1 and 895–903 cm−1 (Fig. 6). The composition of phase X may be inhomogeneous in K/(K + Na), which may range between 0·15 and 0·88 in an individual run (Table 5). This compositional inhomogeneity is restricted to the K/(K + Na) ratio and occurs even if the coexisting phases are well equilibrated. Other chemical characteristics such as Al or Ca contents and Mg/Si ratios show no correlation with K/(K + Na). A decrease in the degree of inhomogeneity can be observed with increasing pressure and K2O contents. Because the inhomogeneity of the phase X composition is independent of run duration and temperature, it cannot be easily explained by a failure of the runs to attain equilibrium. It might instead be a quench effect promoted by high Na contents of phase X. This effect may result from the difference of the ionic radii of K and Na,which leads to a lattice collapse during pressure release. Excluding Na-rich rims, an increase in K2O and a decrease in Na2O with increasing P (and T) is observed (Fig. 7). In the subalkaline bulk composition, the highest K2O contents of phase X (20–25 wt %) are correlated with the highest oxide totals of >99 wt % (Fig. 8). Although oxide totals can be affected by the analytical technique (i.e. variable electron beam raster size adjusted to grain size of phase X) this correlation indicates decreasing H2O contents of phase X with increasing P. The effect may be explained by an increasing ability of coexisting phases—especially Mg2SiO4—to incorporate H2O (Kohlstedt et al., 1996), which changes H2O partitioning and leads to a continuous dehydration of phase X. The increase in K of phase X with pressure is consistent with other studies (Luth, 1997; Inoue et al., 1998) and parallels results for phlogopite and K-richterite (Konzett & Ulmer, 1999).
Average and representative analyses of phase X
| Exp.: | JKW9 | Ma91B | JKW14 | JKW7 | JKW25 | Ma92B | Ma102M | JKW16 | Ma94sB | JKW29 | JKW64 | JKW67 | |||||
| P (GPa): | 12 | 14 | 14 | 14 | 15 | 15 | 18 | 20 | 15 | 14 | 20 | 20 | |||||
| T (°C): | 1300 | 1100 | 1200 | 1300 | 900 | 1100 | 1300 | 1300 | 1100 | 1100 | 1600 | 1800 | |||||
| Bulk: | per | per | per | per | per | per | per | sub | sub | sub | sub | sub | |||||
| K-rich | Na-rich | K-rich | Na-rich | K-rich | Na-rich | K-rich | Na-rich | K-rich | Na-rich | ||||||||
| No. of | |||||||||||||||||
| analyses: | 6 | 6 | 11 | 11 | 3 | 3 | 7 | 7 | 7 | 9 | 7 | 16 | 15 | 5 | 5 | 3 | |
| SiO2 | 47·2(7) | 46·0 | 48·3(1) | 49·8 | 46·3(5) | 47·8 | 45·7(5) | 44·7 | 44·1(9) | 47·6(8) | 51·1 | 47·9(8) | 47·7(6) | 47·8(8) | 46·4(1) | 45·8(8) | 43·7(9) |
| Al2O3 | 2·5(1) | 2·6 | 2·1(0) | 2·3 | 2·1(1) | 2·3 | 1·9(1) | 1·9 | 2·1(3) | 2·2(2) | 2·2 | 1·4(0) | 2·1(3) | 2·0(2) | 1·9(1) | 1·2(1) | 0·6(1) |
| MgO | 29·3(4) | 28·7 | 30·4(6) | 31·8 | 29·5(3) | 31·7 | 30·3(4) | 28·9 | 28·1(5) | 30·9(6) | 33·1 | 31·2(5) | 30·4(7) | 31·1(4) | 30·3(6) | 29·8(6) | 29·4(3) |
| CaO | 0·2(0) | 0·2 | 0·2(0) | 0·2 | 0·3(0) | 0·4 | 0·3(1) | 0·3 | 0·3(2) | 0·2(1) | 0·3 | 0·5(1) | 0·5(1) | 0·3(1) | 0·2(0) | 0·4(0) | 0·2(1) |
| Na2O | 3·8(3) | 7·6 | 1·7(2) | 7·3 | 2·3(1) | 11·4 | 2·3(3) | 3·5 | 1·1(5) | 2·0(3) | 7·7 | 1·8(3) | 1·1(3) | 2·3(4) | 2·0(2) | 1·5(2) | 1·7(0) |
| K2O | 14·1(1) | 11·2 | 15·1(6) | 2·4 | 16·9(4) | 3·8 | 17·8(9) | 17·6 | 22·8(1) | 15·7(5) | 2·9 | 16·3(6) | 17·4(5) | 14·5(6) | 16·3(1) | 20·8(7) | 25·0(7) |
| ∑ | 97·0(7) | 96·2 | 97·9(1) | 93·8 | 97·3(7) | 97·3 | 98·2(1) | 96·9 | 98·5(9) | 98·5(1) | 97·4 | 99·1(1) | 99·2(7) | 98·0(7) | 96·8(1) | 99·3(7) | 100·5(5) |
| Exp.: | JKW9 | Ma91B | JKW14 | JKW7 | JKW25 | Ma92B | Ma102M | JKW16 | Ma94sB | JKW29 | JKW64 | JKW67 | |||||
| P (GPa): | 12 | 14 | 14 | 14 | 15 | 15 | 18 | 20 | 15 | 14 | 20 | 20 | |||||
| T (°C): | 1300 | 1100 | 1200 | 1300 | 900 | 1100 | 1300 | 1300 | 1100 | 1100 | 1600 | 1800 | |||||
| Bulk: | per | per | per | per | per | per | per | sub | sub | sub | sub | sub | |||||
| K-rich | Na-rich | K-rich | Na-rich | K-rich | Na-rich | K-rich | Na-rich | K-rich | Na-rich | ||||||||
| No. of | |||||||||||||||||
| analyses: | 6 | 6 | 11 | 11 | 3 | 3 | 7 | 7 | 7 | 9 | 7 | 16 | 15 | 5 | 5 | 3 | |
| SiO2 | 47·2(7) | 46·0 | 48·3(1) | 49·8 | 46·3(5) | 47·8 | 45·7(5) | 44·7 | 44·1(9) | 47·6(8) | 51·1 | 47·9(8) | 47·7(6) | 47·8(8) | 46·4(1) | 45·8(8) | 43·7(9) |
| Al2O3 | 2·5(1) | 2·6 | 2·1(0) | 2·3 | 2·1(1) | 2·3 | 1·9(1) | 1·9 | 2·1(3) | 2·2(2) | 2·2 | 1·4(0) | 2·1(3) | 2·0(2) | 1·9(1) | 1·2(1) | 0·6(1) |
| MgO | 29·3(4) | 28·7 | 30·4(6) | 31·8 | 29·5(3) | 31·7 | 30·3(4) | 28·9 | 28·1(5) | 30·9(6) | 33·1 | 31·2(5) | 30·4(7) | 31·1(4) | 30·3(6) | 29·8(6) | 29·4(3) |
| CaO | 0·2(0) | 0·2 | 0·2(0) | 0·2 | 0·3(0) | 0·4 | 0·3(1) | 0·3 | 0·3(2) | 0·2(1) | 0·3 | 0·5(1) | 0·5(1) | 0·3(1) | 0·2(0) | 0·4(0) | 0·2(1) |
| Na2O | 3·8(3) | 7·6 | 1·7(2) | 7·3 | 2·3(1) | 11·4 | 2·3(3) | 3·5 | 1·1(5) | 2·0(3) | 7·7 | 1·8(3) | 1·1(3) | 2·3(4) | 2·0(2) | 1·5(2) | 1·7(0) |
| K2O | 14·1(1) | 11·2 | 15·1(6) | 2·4 | 16·9(4) | 3·8 | 17·8(9) | 17·6 | 22·8(1) | 15·7(5) | 2·9 | 16·3(6) | 17·4(5) | 14·5(6) | 16·3(1) | 20·8(7) | 25·0(7) |
| ∑ | 97·0(7) | 96·2 | 97·9(1) | 93·8 | 97·3(7) | 97·3 | 98·2(1) | 96·9 | 98·5(9) | 98·5(1) | 97·4 | 99·1(1) | 99·2(7) | 98·0(7) | 96·8(1) | 99·3(7) | 100·5(5) |
Average and representative analyses of phase X
| Exp.: | JKW9 | Ma91B | JKW14 | JKW7 | JKW25 | Ma92B | Ma102M | JKW16 | Ma94sB | JKW29 | JKW64 | JKW67 | |||||
| P (GPa): | 12 | 14 | 14 | 14 | 15 | 15 | 18 | 20 | 15 | 14 | 20 | 20 | |||||
| T (°C): | 1300 | 1100 | 1200 | 1300 | 900 | 1100 | 1300 | 1300 | 1100 | 1100 | 1600 | 1800 | |||||
| Bulk: | per | per | per | per | per | per | per | sub | sub | sub | sub | sub | |||||
| K-rich | Na-rich | K-rich | Na-rich | K-rich | Na-rich | K-rich | Na-rich | K-rich | Na-rich | ||||||||
| No. of | |||||||||||||||||
| analyses: | 6 | 6 | 11 | 11 | 3 | 3 | 7 | 7 | 7 | 9 | 7 | 16 | 15 | 5 | 5 | 3 | |
| SiO2 | 47·2(7) | 46·0 | 48·3(1) | 49·8 | 46·3(5) | 47·8 | 45·7(5) | 44·7 | 44·1(9) | 47·6(8) | 51·1 | 47·9(8) | 47·7(6) | 47·8(8) | 46·4(1) | 45·8(8) | 43·7(9) |
| Al2O3 | 2·5(1) | 2·6 | 2·1(0) | 2·3 | 2·1(1) | 2·3 | 1·9(1) | 1·9 | 2·1(3) | 2·2(2) | 2·2 | 1·4(0) | 2·1(3) | 2·0(2) | 1·9(1) | 1·2(1) | 0·6(1) |
| MgO | 29·3(4) | 28·7 | 30·4(6) | 31·8 | 29·5(3) | 31·7 | 30·3(4) | 28·9 | 28·1(5) | 30·9(6) | 33·1 | 31·2(5) | 30·4(7) | 31·1(4) | 30·3(6) | 29·8(6) | 29·4(3) |
| CaO | 0·2(0) | 0·2 | 0·2(0) | 0·2 | 0·3(0) | 0·4 | 0·3(1) | 0·3 | 0·3(2) | 0·2(1) | 0·3 | 0·5(1) | 0·5(1) | 0·3(1) | 0·2(0) | 0·4(0) | 0·2(1) |
| Na2O | 3·8(3) | 7·6 | 1·7(2) | 7·3 | 2·3(1) | 11·4 | 2·3(3) | 3·5 | 1·1(5) | 2·0(3) | 7·7 | 1·8(3) | 1·1(3) | 2·3(4) | 2·0(2) | 1·5(2) | 1·7(0) |
| K2O | 14·1(1) | 11·2 | 15·1(6) | 2·4 | 16·9(4) | 3·8 | 17·8(9) | 17·6 | 22·8(1) | 15·7(5) | 2·9 | 16·3(6) | 17·4(5) | 14·5(6) | 16·3(1) | 20·8(7) | 25·0(7) |
| ∑ | 97·0(7) | 96·2 | 97·9(1) | 93·8 | 97·3(7) | 97·3 | 98·2(1) | 96·9 | 98·5(9) | 98·5(1) | 97·4 | 99·1(1) | 99·2(7) | 98·0(7) | 96·8(1) | 99·3(7) | 100·5(5) |
| Exp.: | JKW9 | Ma91B | JKW14 | JKW7 | JKW25 | Ma92B | Ma102M | JKW16 | Ma94sB | JKW29 | JKW64 | JKW67 | |||||
| P (GPa): | 12 | 14 | 14 | 14 | 15 | 15 | 18 | 20 | 15 | 14 | 20 | 20 | |||||
| T (°C): | 1300 | 1100 | 1200 | 1300 | 900 | 1100 | 1300 | 1300 | 1100 | 1100 | 1600 | 1800 | |||||
| Bulk: | per | per | per | per | per | per | per | sub | sub | sub | sub | sub | |||||
| K-rich | Na-rich | K-rich | Na-rich | K-rich | Na-rich | K-rich | Na-rich | K-rich | Na-rich | ||||||||
| No. of | |||||||||||||||||
| analyses: | 6 | 6 | 11 | 11 | 3 | 3 | 7 | 7 | 7 | 9 | 7 | 16 | 15 | 5 | 5 | 3 | |
| SiO2 | 47·2(7) | 46·0 | 48·3(1) | 49·8 | 46·3(5) | 47·8 | 45·7(5) | 44·7 | 44·1(9) | 47·6(8) | 51·1 | 47·9(8) | 47·7(6) | 47·8(8) | 46·4(1) | 45·8(8) | 43·7(9) |
| Al2O3 | 2·5(1) | 2·6 | 2·1(0) | 2·3 | 2·1(1) | 2·3 | 1·9(1) | 1·9 | 2·1(3) | 2·2(2) | 2·2 | 1·4(0) | 2·1(3) | 2·0(2) | 1·9(1) | 1·2(1) | 0·6(1) |
| MgO | 29·3(4) | 28·7 | 30·4(6) | 31·8 | 29·5(3) | 31·7 | 30·3(4) | 28·9 | 28·1(5) | 30·9(6) | 33·1 | 31·2(5) | 30·4(7) | 31·1(4) | 30·3(6) | 29·8(6) | 29·4(3) |
| CaO | 0·2(0) | 0·2 | 0·2(0) | 0·2 | 0·3(0) | 0·4 | 0·3(1) | 0·3 | 0·3(2) | 0·2(1) | 0·3 | 0·5(1) | 0·5(1) | 0·3(1) | 0·2(0) | 0·4(0) | 0·2(1) |
| Na2O | 3·8(3) | 7·6 | 1·7(2) | 7·3 | 2·3(1) | 11·4 | 2·3(3) | 3·5 | 1·1(5) | 2·0(3) | 7·7 | 1·8(3) | 1·1(3) | 2·3(4) | 2·0(2) | 1·5(2) | 1·7(0) |
| K2O | 14·1(1) | 11·2 | 15·1(6) | 2·4 | 16·9(4) | 3·8 | 17·8(9) | 17·6 | 22·8(1) | 15·7(5) | 2·9 | 16·3(6) | 17·4(5) | 14·5(6) | 16·3(1) | 20·8(7) | 25·0(7) |
| ∑ | 97·0(7) | 96·2 | 97·9(1) | 93·8 | 97·3(7) | 97·3 | 98·2(1) | 96·9 | 98·5(9) | 98·5(1) | 97·4 | 99·1(1) | 99·2(7) | 98·0(7) | 96·8(1) | 99·3(7) | 100·5(5) |
Unoriented laser Raman spectrum of phase X from run JKW7 (14 GPa and 1300°C, peralkaline KNCMASH) in the silicate (200–1200 cm−1) and the OH-stretching region (3500–3750 cm−1).
Selected mineral chemical parameters of phase X as a function of P and T. •, Runs at 1300°C; ♦, runs at 1100°C; □, runs at 1200°C; ×, runs at 900°C; ▪, runs at 1700°C; ▴, runs at 1600°C; open bars, composition range of phase X (Luth,1997); open stars, compositions of phase X obtained by Inoue et al. (1998); in (e) and (g), ○ and • are analyses of K-rich and Na-rich grains/grain areas of phase X (see Table 5), respectively; in (a) and (c), only K-rich phase X analyses are plotted.
Plot of wt % (K2O + Na2O) vs oxide totals obtained from electron microprobe analyses for phase X from the subalkaline KNCMASH system. Only K-rich phase X analyses are plotted.
Phase X contains from 0·4 to 2·6 wt % Al2O3 and from 0·05 to 0·5 wt % CaO, respectively, values consistent with observations by Luth (1997) and Inoue et al. (1998). Al decreases with increasing P at constant T, but varies little with T (Fig. 7). The increase of Al in phase X in the peralkaline bulk composition at 20 GPa reflects the stabilization of K-hollandite. The molar Si/(Mg + Al) ratios of individual phase X analyses are consistently closer to 1·0 than are the Si/Mg ratios. This suggests a replacement of six-coordinated Mg by Al through a coupled substitution to maintain charge balance. Although there are several possibilities, such as □Al2Mg−3 or AlAlMg−1Si−1, the structure analysis gives no indication of [IV]Al and the most likely substitution is Al□Mg−1K−1. This exchange introduces a further vacancy on the K position for charge balance and would lead to a theoretical phase X end-member □2(MgAl)Si2O7H or □K(MgAl)Si2O7 for the anhydrous end-member. The Ca content of phase X does not exceed 0·8 wt % (Fig. 7) and can be explained by a simple CaMg−1 exchange.
Garnet
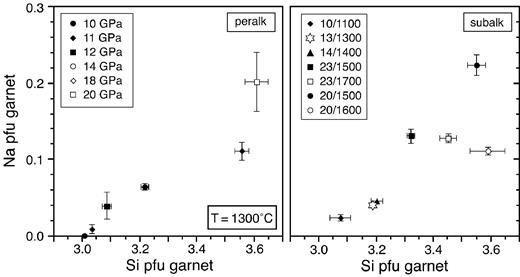
Plot of Na p.f.u. vs Si p.f.u. for garnets from the subalkaline and peralkaline KNCMASH systems.
Average analyses of garnet
| Exp.: | JKW19 | JKW13 | JKW15 | JKW18 | JKW17 | Ma88sB | JKW29 | JKW33 | JKW30 | JKW41 | JKW47 | JKW64 | JKW61 | JKW54 |
| Bulk: | per | per | per | per | per | per | sub | sub | sub | sub | sub | sub | sub | sub |
| P (GPa): | 10 | 10 | 10 | 10 | 11 | 13 | 14 | 14 | 13 | 13 | 20 | 20 | 23 | 23 |
| T (°C): | 1100 | 1200 | 1300 | 1350 | 1300 | 1100 | 1100 | 1400 | 1100 | 1300 | 1500 | 1600 | 1500 | 1700 |
| No. of analyses: | 6 | 7 | 7 | 5 | 8 | 9 | 7 | 6 | 5 | 5 | 5 | 10 | 11 | 10 |
| SiO2 | 42·5(3) | 42·3(4) | 42·1(3) | 43·7(2) | 43·4(4) | 46·6(4) | 47·8(3) | 46·6(5) | 46·9(3) | 46·1(2) | 51·6(1) | 53·2(9) | 49·6(3) | 51·6(4) |
| Al2O3 | 23·1(3) | 23·3(2) | 22·9(2) | 23·2(1) | 23·2(2) | 22·2(9) | 19·7(5) | 19·8(5) | 21·6(2) | 20·0(1) | 13·4(5) | 11·1(9) | 18·7(3) | 15·5(7) |
| MgO | 19·3(8) | 18·1(6) | 15·8(3) | 23·0(5) | 21·1(7) | 25·4(5) | 26·2(5) | 26·9(6) | 25·6(4) | 26·4(3) | 26·4(4) | 29·6(8) | 28·3(2) | 29·5(3) |
| CaO | 13·7(9) | 14·9(9) | 18·2(4) | 9·3(6) | 11·4(9) | 7·2(9) | 6·6(4) | 5·7(4) | 6·9(3) | 6·0(1) | 6·1(2) | 6·4(4) | 3·4(2) | 3·7(2) |
| Na2O | 0·1(1) | 0·1(0) | <0·05 | 0·1(0) | 0·1(1) | 0·4(1) | 0·5(1) | 0·3(0) | 0·3(0) | 0·3(0) | 1·7(1) | 0·9(0) | 1·0(1) | 1·0(0) |
| K2O | <0·05 | <0·05 | <0·05 | <0·05 | <0·05 | <0·05 | <0·05 | <0·05 | <0·05 | <0·05 | <0·05 | |||
| ∑ | 98·7(6) | 98·7(6) | 99·0(6) | 99·4(2) | 99·1(5) | 101·8(5) | 100·9(4) | 99·3(5) | 101·2(4) | 98·9(4) | 99·2(1) | 101·2(5) | 101·0(4) | 101·3(3) |
| Si | 3·01(2) | 3·01(1) | 3·01(1) | 3·03(1) | 3·06(1) | 3·13(3) | 3·24(1) | 3·20(2) | 3·17(1) | 3·19(1) | 3·55(3) | 3·59(6) | 3·32(1) | 3·45(3) |
| Al | 1·93(2) | 1·95(2) | 1·93(1) | 1·90(1) | 1·91(1) | 1·76(7) | 1·58(4) | 1·60(5) | 1·72(2) | 1·63(1) | 1·09(5) | 0·89(1) | 1·48(2) | 1·22(5) |
| Mg | 2·04(7) | 1·92(6) | 1·69(4) | 2·39(5) | 2·21(7) | 2·55(5) | 2·64(5) | 2·75(5) | 2·57(3) | 2·71(2) | 2·71(1) | 2·98(8) | 2·83(2) | 2·94(3) |
| Ca | 1·04(8) | 1·14(7) | 1·40(3) | 0·69(5) | 0·85(7) | 0·52(7) | 0·48(3) | 0·42(3) | 0·50(2) | 0·45(1) | 0·45(1) | 0·46(3) | 0·24(1) | 0·27(1) |
| Na | 0·01(1) | 0·01(0) | — | 0·01(0) | 0·01(1) | 0·05(1) | 0·07(1) | 0·05(0) | 0·04(0) | 0·04(0) | 0·22(1) | 0·11(1) | 0·13(1) | 0·13(1) |
| ∑ | 8·03(2) | 8·02(0) | 8·03(1) | 8·03(1) | 8·01(1) | 8·01(1) | 8·01(2) | 8·02(1) | 7·99(1) | 8·02(1) | 8·02(1) | 8·02(1) | 8·00(1) | 8·00(1) |
| Ca/(Ca+Mg) | 0·34(2) | 0·37(2) | 0·45(1) | 0·22(1) | 0·28(2) | 0·17(1) | 0·15(1) | 0·13(1) | 0·16(1) | 0·14(0) | 0·14(0) | 0·14(1) | 0·11(1) | 0·11(1) |
| ppm K | — | — | — | — | — | — | — | — | — | — | — | 296(102) | 449(157) | 728(375) |
| Exp.: | JKW19 | JKW13 | JKW15 | JKW18 | JKW17 | Ma88sB | JKW29 | JKW33 | JKW30 | JKW41 | JKW47 | JKW64 | JKW61 | JKW54 |
| Bulk: | per | per | per | per | per | per | sub | sub | sub | sub | sub | sub | sub | sub |
| P (GPa): | 10 | 10 | 10 | 10 | 11 | 13 | 14 | 14 | 13 | 13 | 20 | 20 | 23 | 23 |
| T (°C): | 1100 | 1200 | 1300 | 1350 | 1300 | 1100 | 1100 | 1400 | 1100 | 1300 | 1500 | 1600 | 1500 | 1700 |
| No. of analyses: | 6 | 7 | 7 | 5 | 8 | 9 | 7 | 6 | 5 | 5 | 5 | 10 | 11 | 10 |
| SiO2 | 42·5(3) | 42·3(4) | 42·1(3) | 43·7(2) | 43·4(4) | 46·6(4) | 47·8(3) | 46·6(5) | 46·9(3) | 46·1(2) | 51·6(1) | 53·2(9) | 49·6(3) | 51·6(4) |
| Al2O3 | 23·1(3) | 23·3(2) | 22·9(2) | 23·2(1) | 23·2(2) | 22·2(9) | 19·7(5) | 19·8(5) | 21·6(2) | 20·0(1) | 13·4(5) | 11·1(9) | 18·7(3) | 15·5(7) |
| MgO | 19·3(8) | 18·1(6) | 15·8(3) | 23·0(5) | 21·1(7) | 25·4(5) | 26·2(5) | 26·9(6) | 25·6(4) | 26·4(3) | 26·4(4) | 29·6(8) | 28·3(2) | 29·5(3) |
| CaO | 13·7(9) | 14·9(9) | 18·2(4) | 9·3(6) | 11·4(9) | 7·2(9) | 6·6(4) | 5·7(4) | 6·9(3) | 6·0(1) | 6·1(2) | 6·4(4) | 3·4(2) | 3·7(2) |
| Na2O | 0·1(1) | 0·1(0) | <0·05 | 0·1(0) | 0·1(1) | 0·4(1) | 0·5(1) | 0·3(0) | 0·3(0) | 0·3(0) | 1·7(1) | 0·9(0) | 1·0(1) | 1·0(0) |
| K2O | <0·05 | <0·05 | <0·05 | <0·05 | <0·05 | <0·05 | <0·05 | <0·05 | <0·05 | <0·05 | <0·05 | |||
| ∑ | 98·7(6) | 98·7(6) | 99·0(6) | 99·4(2) | 99·1(5) | 101·8(5) | 100·9(4) | 99·3(5) | 101·2(4) | 98·9(4) | 99·2(1) | 101·2(5) | 101·0(4) | 101·3(3) |
| Si | 3·01(2) | 3·01(1) | 3·01(1) | 3·03(1) | 3·06(1) | 3·13(3) | 3·24(1) | 3·20(2) | 3·17(1) | 3·19(1) | 3·55(3) | 3·59(6) | 3·32(1) | 3·45(3) |
| Al | 1·93(2) | 1·95(2) | 1·93(1) | 1·90(1) | 1·91(1) | 1·76(7) | 1·58(4) | 1·60(5) | 1·72(2) | 1·63(1) | 1·09(5) | 0·89(1) | 1·48(2) | 1·22(5) |
| Mg | 2·04(7) | 1·92(6) | 1·69(4) | 2·39(5) | 2·21(7) | 2·55(5) | 2·64(5) | 2·75(5) | 2·57(3) | 2·71(2) | 2·71(1) | 2·98(8) | 2·83(2) | 2·94(3) |
| Ca | 1·04(8) | 1·14(7) | 1·40(3) | 0·69(5) | 0·85(7) | 0·52(7) | 0·48(3) | 0·42(3) | 0·50(2) | 0·45(1) | 0·45(1) | 0·46(3) | 0·24(1) | 0·27(1) |
| Na | 0·01(1) | 0·01(0) | — | 0·01(0) | 0·01(1) | 0·05(1) | 0·07(1) | 0·05(0) | 0·04(0) | 0·04(0) | 0·22(1) | 0·11(1) | 0·13(1) | 0·13(1) |
| ∑ | 8·03(2) | 8·02(0) | 8·03(1) | 8·03(1) | 8·01(1) | 8·01(1) | 8·01(2) | 8·02(1) | 7·99(1) | 8·02(1) | 8·02(1) | 8·02(1) | 8·00(1) | 8·00(1) |
| Ca/(Ca+Mg) | 0·34(2) | 0·37(2) | 0·45(1) | 0·22(1) | 0·28(2) | 0·17(1) | 0·15(1) | 0·13(1) | 0·16(1) | 0·14(0) | 0·14(0) | 0·14(1) | 0·11(1) | 0·11(1) |
| ppm K | — | — | — | — | — | — | — | — | — | — | — | 296(102) | 449(157) | 728(375) |
Garnet formulae recalculated to 12 oxygens. Lower limit of detection for K: JKW54 240 ppm; JKW61 230 ppm; JKW64: 190 ppm.
Average analyses of garnet
| Exp.: | JKW19 | JKW13 | JKW15 | JKW18 | JKW17 | Ma88sB | JKW29 | JKW33 | JKW30 | JKW41 | JKW47 | JKW64 | JKW61 | JKW54 |
| Bulk: | per | per | per | per | per | per | sub | sub | sub | sub | sub | sub | sub | sub |
| P (GPa): | 10 | 10 | 10 | 10 | 11 | 13 | 14 | 14 | 13 | 13 | 20 | 20 | 23 | 23 |
| T (°C): | 1100 | 1200 | 1300 | 1350 | 1300 | 1100 | 1100 | 1400 | 1100 | 1300 | 1500 | 1600 | 1500 | 1700 |
| No. of analyses: | 6 | 7 | 7 | 5 | 8 | 9 | 7 | 6 | 5 | 5 | 5 | 10 | 11 | 10 |
| SiO2 | 42·5(3) | 42·3(4) | 42·1(3) | 43·7(2) | 43·4(4) | 46·6(4) | 47·8(3) | 46·6(5) | 46·9(3) | 46·1(2) | 51·6(1) | 53·2(9) | 49·6(3) | 51·6(4) |
| Al2O3 | 23·1(3) | 23·3(2) | 22·9(2) | 23·2(1) | 23·2(2) | 22·2(9) | 19·7(5) | 19·8(5) | 21·6(2) | 20·0(1) | 13·4(5) | 11·1(9) | 18·7(3) | 15·5(7) |
| MgO | 19·3(8) | 18·1(6) | 15·8(3) | 23·0(5) | 21·1(7) | 25·4(5) | 26·2(5) | 26·9(6) | 25·6(4) | 26·4(3) | 26·4(4) | 29·6(8) | 28·3(2) | 29·5(3) |
| CaO | 13·7(9) | 14·9(9) | 18·2(4) | 9·3(6) | 11·4(9) | 7·2(9) | 6·6(4) | 5·7(4) | 6·9(3) | 6·0(1) | 6·1(2) | 6·4(4) | 3·4(2) | 3·7(2) |
| Na2O | 0·1(1) | 0·1(0) | <0·05 | 0·1(0) | 0·1(1) | 0·4(1) | 0·5(1) | 0·3(0) | 0·3(0) | 0·3(0) | 1·7(1) | 0·9(0) | 1·0(1) | 1·0(0) |
| K2O | <0·05 | <0·05 | <0·05 | <0·05 | <0·05 | <0·05 | <0·05 | <0·05 | <0·05 | <0·05 | <0·05 | |||
| ∑ | 98·7(6) | 98·7(6) | 99·0(6) | 99·4(2) | 99·1(5) | 101·8(5) | 100·9(4) | 99·3(5) | 101·2(4) | 98·9(4) | 99·2(1) | 101·2(5) | 101·0(4) | 101·3(3) |
| Si | 3·01(2) | 3·01(1) | 3·01(1) | 3·03(1) | 3·06(1) | 3·13(3) | 3·24(1) | 3·20(2) | 3·17(1) | 3·19(1) | 3·55(3) | 3·59(6) | 3·32(1) | 3·45(3) |
| Al | 1·93(2) | 1·95(2) | 1·93(1) | 1·90(1) | 1·91(1) | 1·76(7) | 1·58(4) | 1·60(5) | 1·72(2) | 1·63(1) | 1·09(5) | 0·89(1) | 1·48(2) | 1·22(5) |
| Mg | 2·04(7) | 1·92(6) | 1·69(4) | 2·39(5) | 2·21(7) | 2·55(5) | 2·64(5) | 2·75(5) | 2·57(3) | 2·71(2) | 2·71(1) | 2·98(8) | 2·83(2) | 2·94(3) |
| Ca | 1·04(8) | 1·14(7) | 1·40(3) | 0·69(5) | 0·85(7) | 0·52(7) | 0·48(3) | 0·42(3) | 0·50(2) | 0·45(1) | 0·45(1) | 0·46(3) | 0·24(1) | 0·27(1) |
| Na | 0·01(1) | 0·01(0) | — | 0·01(0) | 0·01(1) | 0·05(1) | 0·07(1) | 0·05(0) | 0·04(0) | 0·04(0) | 0·22(1) | 0·11(1) | 0·13(1) | 0·13(1) |
| ∑ | 8·03(2) | 8·02(0) | 8·03(1) | 8·03(1) | 8·01(1) | 8·01(1) | 8·01(2) | 8·02(1) | 7·99(1) | 8·02(1) | 8·02(1) | 8·02(1) | 8·00(1) | 8·00(1) |
| Ca/(Ca+Mg) | 0·34(2) | 0·37(2) | 0·45(1) | 0·22(1) | 0·28(2) | 0·17(1) | 0·15(1) | 0·13(1) | 0·16(1) | 0·14(0) | 0·14(0) | 0·14(1) | 0·11(1) | 0·11(1) |
| ppm K | — | — | — | — | — | — | — | — | — | — | — | 296(102) | 449(157) | 728(375) |
| Exp.: | JKW19 | JKW13 | JKW15 | JKW18 | JKW17 | Ma88sB | JKW29 | JKW33 | JKW30 | JKW41 | JKW47 | JKW64 | JKW61 | JKW54 |
| Bulk: | per | per | per | per | per | per | sub | sub | sub | sub | sub | sub | sub | sub |
| P (GPa): | 10 | 10 | 10 | 10 | 11 | 13 | 14 | 14 | 13 | 13 | 20 | 20 | 23 | 23 |
| T (°C): | 1100 | 1200 | 1300 | 1350 | 1300 | 1100 | 1100 | 1400 | 1100 | 1300 | 1500 | 1600 | 1500 | 1700 |
| No. of analyses: | 6 | 7 | 7 | 5 | 8 | 9 | 7 | 6 | 5 | 5 | 5 | 10 | 11 | 10 |
| SiO2 | 42·5(3) | 42·3(4) | 42·1(3) | 43·7(2) | 43·4(4) | 46·6(4) | 47·8(3) | 46·6(5) | 46·9(3) | 46·1(2) | 51·6(1) | 53·2(9) | 49·6(3) | 51·6(4) |
| Al2O3 | 23·1(3) | 23·3(2) | 22·9(2) | 23·2(1) | 23·2(2) | 22·2(9) | 19·7(5) | 19·8(5) | 21·6(2) | 20·0(1) | 13·4(5) | 11·1(9) | 18·7(3) | 15·5(7) |
| MgO | 19·3(8) | 18·1(6) | 15·8(3) | 23·0(5) | 21·1(7) | 25·4(5) | 26·2(5) | 26·9(6) | 25·6(4) | 26·4(3) | 26·4(4) | 29·6(8) | 28·3(2) | 29·5(3) |
| CaO | 13·7(9) | 14·9(9) | 18·2(4) | 9·3(6) | 11·4(9) | 7·2(9) | 6·6(4) | 5·7(4) | 6·9(3) | 6·0(1) | 6·1(2) | 6·4(4) | 3·4(2) | 3·7(2) |
| Na2O | 0·1(1) | 0·1(0) | <0·05 | 0·1(0) | 0·1(1) | 0·4(1) | 0·5(1) | 0·3(0) | 0·3(0) | 0·3(0) | 1·7(1) | 0·9(0) | 1·0(1) | 1·0(0) |
| K2O | <0·05 | <0·05 | <0·05 | <0·05 | <0·05 | <0·05 | <0·05 | <0·05 | <0·05 | <0·05 | <0·05 | |||
| ∑ | 98·7(6) | 98·7(6) | 99·0(6) | 99·4(2) | 99·1(5) | 101·8(5) | 100·9(4) | 99·3(5) | 101·2(4) | 98·9(4) | 99·2(1) | 101·2(5) | 101·0(4) | 101·3(3) |
| Si | 3·01(2) | 3·01(1) | 3·01(1) | 3·03(1) | 3·06(1) | 3·13(3) | 3·24(1) | 3·20(2) | 3·17(1) | 3·19(1) | 3·55(3) | 3·59(6) | 3·32(1) | 3·45(3) |
| Al | 1·93(2) | 1·95(2) | 1·93(1) | 1·90(1) | 1·91(1) | 1·76(7) | 1·58(4) | 1·60(5) | 1·72(2) | 1·63(1) | 1·09(5) | 0·89(1) | 1·48(2) | 1·22(5) |
| Mg | 2·04(7) | 1·92(6) | 1·69(4) | 2·39(5) | 2·21(7) | 2·55(5) | 2·64(5) | 2·75(5) | 2·57(3) | 2·71(2) | 2·71(1) | 2·98(8) | 2·83(2) | 2·94(3) |
| Ca | 1·04(8) | 1·14(7) | 1·40(3) | 0·69(5) | 0·85(7) | 0·52(7) | 0·48(3) | 0·42(3) | 0·50(2) | 0·45(1) | 0·45(1) | 0·46(3) | 0·24(1) | 0·27(1) |
| Na | 0·01(1) | 0·01(0) | — | 0·01(0) | 0·01(1) | 0·05(1) | 0·07(1) | 0·05(0) | 0·04(0) | 0·04(0) | 0·22(1) | 0·11(1) | 0·13(1) | 0·13(1) |
| ∑ | 8·03(2) | 8·02(0) | 8·03(1) | 8·03(1) | 8·01(1) | 8·01(1) | 8·01(2) | 8·02(1) | 7·99(1) | 8·02(1) | 8·02(1) | 8·02(1) | 8·00(1) | 8·00(1) |
| Ca/(Ca+Mg) | 0·34(2) | 0·37(2) | 0·45(1) | 0·22(1) | 0·28(2) | 0·17(1) | 0·15(1) | 0·13(1) | 0·16(1) | 0·14(0) | 0·14(0) | 0·14(1) | 0·11(1) | 0·11(1) |
| ppm K | — | — | — | — | — | — | — | — | — | — | — | 296(102) | 449(157) | 728(375) |
Garnet formulae recalculated to 12 oxygens. Lower limit of detection for K: JKW54 240 ppm; JKW61 230 ppm; JKW64: 190 ppm.
High-Ca clinopyroxene
High-Ca clinopyroxene is essentially a binary diopside–jadeite solid solution with <0·25 Na p.f.u. and minor amounts of Mg(M2) in most cases (Table 7). Na varies little between 10 and 15 GPa but decreases strongly near the upper P stability limit of clinopyroxene to 0·06 Na p.f.u., equivalent to 5 mol % jadeite component. This reflects a continuous reaction of jadeite component to form Na-garnet (see above). Averaged K contents range between 0·2 and 0·8 wt % K2O. These lack systematic trends with either P or T, instead reflecting the strong dependence of K in high-Ca clinopyroxene upon the coexisting assemblage (Luth, 1997).
Average analyses of high-Ca clinopyroxene
| Exp.: | JKW19 | JKW13 | JKW15 | JKW18 | JKW17 | JKW9 | Ma88sB | Ma91B | Ma92B | Ma102M | Ma104M | JKW7 | JKW14 | JKW25 | Ma95sB | Ma94sB | JKW29 | JKW41 | JKW33 | JKW34 | JKW30 |
| Bulk: | per | per | per | per | per | per | per | per | per | per | per | per | per | per | sub | sub | sub | sub | sub | sub | sub |
| P (GPa): | 10 | 10 | 10 | 10 | 11 | 12 | 13 | 14 | 15 | 18 | 13 | 14 | 14 | 15 | 10 | 15 | 14 | 13 | 14 | 14 | 13 |
| T (°C): | 1100 | 1200 | 1300 | 1350 | 1300 | 1300 | 1100 | 1100 | 1100 | 1300 | 1400 | 1300 | 1200 | 900 | 1100 | 1100 | 1100 | 1300 | 1400 | 900 | 1100 |
| No. of analyses: | 8 | 12 | 7 | 8 | 8 | 7 | 10 | 12 | 11 | 4 | 4 | 6 | 11 | 5 | 6 | 5 | 9 | 6 | 7 | 6 | 5 |
| SiO2 | 55·4(4) | 55·4(4) | 55·2(3) | 55·2(5) | 55·0(2) | 55·7(1) | 56·4(2) | 55·8(5) | 56·8(2) | 56·1(7) | 57·5(5) | 55·8(4) | 55·4(6) | 56·0(5) | 55·8(2) | 56·8(3) | 56·8(3) | 56·1(2) | 56·0(3) | 55·7 | 55·6(2) |
| Al2O3 | 3·9(5) | 3·2(6) | 2·8(5) | 4·4(7) | 3·3(3) | 3·8(8) | 3·5(5) | 3·8(4) | 3·9(5) | 1·2(4) | 2·8(1) | 3·2(3) | 2·5(5) | 0·4(3) | 2·8(4) | 3·7(5) | 3·3(3) | 3·3(2) | 2·9(2) | 4·9 | 2·4(3) |
| MgO | 16·2(5) | 16·7(5) | 16·8(5) | 16·4(7) | 17·1(2) | 16·9(4) | 17·7(6) | 17·0(4) | 17·6(3) | 19·2(6) | 22·3(3) | 18·2(4) | 17·6(6) | 19·3(7) | 18·4(5) | 18·2(7) | 17·3(5) | 17·8(2) | 18·6(2) | 15·4 | 17·6(2) |
| CaO | 21·0(5) | 21·7(8) | 22·3(5) | 19·7(6) | 21·2(2) | 19·7(7) | 20·3(3) | 20·2(4) | 19·5(7) | 22·0(6) | 16·4(3) | 20·0(2) | 21·4(6) | 24·3(6) | 21·1(4) | 19·0(8) | 20·5(4) | 19·0(2) | 19·6(5) | 18·4 | 21·3(5) |
| Na2O | 2·5(3) | 1·8(4) | 1·6(3) | 2·6(5) | 1·9(1) | 2·2(2) | 2·2(2) | 2·5(2) | 2·6(4) | 0·9(2) | 1·6(1) | 2·1(2) | 1·7(4) | 0·3(3) | 1·6(2) | 2·5(4) | 2·2(2) | 2·0(2) | 1·8(2) | 3·2 | 1·6(2) |
| K2O | 0·2(1) | 0·3(1) | 0·3(0) | 0·5(1) | 0·5(0) | 0·8(1) | 0·5(1) | 0·5(1) | 0·4(1) | 0·6(1) | 0·5(0) | 0·4(0) | 0·4(1) | 0·2(0) | 0·3(0) | 0·4(1) | 0·3(1) | 0·7(3) | 0·6(0) | 0·6 | 0·4(1) |
| ∑ | 99·2(6) | 99·0(4) | 99·0(4) | 98·8(6) | 98·9(3) | 99·1(4) | 100·6(5) | 99·8(7) | 100·8(3) | 100·0(8) | 101·0(6) | 99·9(3) | 99·0(7) | 100·5(8) | 100·0(3) | 100·6(4) | 100·3(4) | 98·9(3) | 99·6(6) | 98·2 | 98·9(3) |
| Si | 1·99(1) | 2·00(1) | 2·00(1) | 1·99(0) | 1·99(0) | 2·00(1) | 2·00(0) | 1·99(0) | 2·00(0) | 2·01(1) | 2·00(1) | 1·99(1) | 2·00(1) | 2·00(1) | 1·99(1) | 2·00(1) | 2·01(0) | 2·01(0) | 2·00(0) | 2·01 | 2·01(0) |
| Al | 0·17(2) | 0·13(3) | 0·12(2) | 0·18(3) | 0·14(1) | 0·16(2) | 0·15(2) | 0·16(2) | 0·16(2) | 0·05(2) | 0·11(0) | 0·14(1) | 0·11(2) | 0·02(2) | 0·12(2) | 0·15(2) | 0·14(1) | 0·14(1) | 0·12(1) | 0·21 | 0·10(1) |
| Mg | 0·87(3) | 0·90(3) | 0·91(3) | 0·88(4) | 0·92(1) | 0·91(2) | 0·93(3) | 0·90(3) | 0·93(2) | 1·02(3) | 1·16(2) | 0·97(2) | 0·95(3) | 1·03(4) | 0·98(3) | 0·96(4) | 0·91(3) | 0·95(1) | 0·99(1) | 0·83 | 0·95(1) |
| Ca | 0·81(2) | 0·84(3) | 0·86(2) | 0·76(3) | 0·82(1) | 0·76(2) | 0·77(1) | 0·77(2) | 0·74(3) | 0·84(2) | 0·61(1) | 0·77(1) | 0·83(2) | 0·93(3) | 0·80(2) | 0·72(3) | 0·78(2) | 0·73(1) | 0·75(2) | 0·71 | 0·82(2) |
| Na | 0·17(2) | 0·12(3) | 0·11(2) | 0·18(3) | 0·13(1) | 0·16(2) | 0·15(2) | 0·17(2) | 0·18(2) | 0·06(1) | 0·11(0) | 0·14(2) | 0·12(3) | 0·02(2) | 0·11(1) | 0·17(3) | 0·15(2) | 0·14(1) | 0·13(1) | 0·22 | 0·11(1) |
| K | 0·01(0) | 0·02(0) | 0·01(0) | 0·02(1) | 0·02(0) | 0·04(1) | 0·02(0) | 0·02(3) | 0·02(2) | 0·03(0) | 0·02(0) | 0·02(0) | 0·02(0) | 0·01(0) | 0·01(1) | 0·02(1) | 0·01(0) | 0·03(0) | 0·03(0) | 0·03 | 0·02(0) |
| ∑ | 4·02(1) | 4·01(1) | 4·01(0) | 4·02(0) | 4·01(0) | 4·01(1) | 4·02(1) | 4·02(5) | 4·02(2) | 4·01(1) | 4·01(1) | 4·02(1) | 4·01(1) | 4·01(1) | 4·02(1) | 4·02(0) | 4·00(1) | 4·00(1) | 4·02(1) | 4·01 | 4·01(0) |
| K/(K+Na) | 0·06(1) | 0·11(2) | 0·09(0) | 0·11(2) | 0·14(1) | 0·19(4) | 0·14(1) | 0·10(1) | 0·09(2) | 0·31(2) | 0·16(1) | 0·10(1) | 0·15(4) | 0·34(2) | 0·11(1) | 0·10(2) | 0·08(1) | 0·18(1) | 0·19(1) | 0·12 | 0·15(1) |
| Exp.: | JKW19 | JKW13 | JKW15 | JKW18 | JKW17 | JKW9 | Ma88sB | Ma91B | Ma92B | Ma102M | Ma104M | JKW7 | JKW14 | JKW25 | Ma95sB | Ma94sB | JKW29 | JKW41 | JKW33 | JKW34 | JKW30 |
| Bulk: | per | per | per | per | per | per | per | per | per | per | per | per | per | per | sub | sub | sub | sub | sub | sub | sub |
| P (GPa): | 10 | 10 | 10 | 10 | 11 | 12 | 13 | 14 | 15 | 18 | 13 | 14 | 14 | 15 | 10 | 15 | 14 | 13 | 14 | 14 | 13 |
| T (°C): | 1100 | 1200 | 1300 | 1350 | 1300 | 1300 | 1100 | 1100 | 1100 | 1300 | 1400 | 1300 | 1200 | 900 | 1100 | 1100 | 1100 | 1300 | 1400 | 900 | 1100 |
| No. of analyses: | 8 | 12 | 7 | 8 | 8 | 7 | 10 | 12 | 11 | 4 | 4 | 6 | 11 | 5 | 6 | 5 | 9 | 6 | 7 | 6 | 5 |
| SiO2 | 55·4(4) | 55·4(4) | 55·2(3) | 55·2(5) | 55·0(2) | 55·7(1) | 56·4(2) | 55·8(5) | 56·8(2) | 56·1(7) | 57·5(5) | 55·8(4) | 55·4(6) | 56·0(5) | 55·8(2) | 56·8(3) | 56·8(3) | 56·1(2) | 56·0(3) | 55·7 | 55·6(2) |
| Al2O3 | 3·9(5) | 3·2(6) | 2·8(5) | 4·4(7) | 3·3(3) | 3·8(8) | 3·5(5) | 3·8(4) | 3·9(5) | 1·2(4) | 2·8(1) | 3·2(3) | 2·5(5) | 0·4(3) | 2·8(4) | 3·7(5) | 3·3(3) | 3·3(2) | 2·9(2) | 4·9 | 2·4(3) |
| MgO | 16·2(5) | 16·7(5) | 16·8(5) | 16·4(7) | 17·1(2) | 16·9(4) | 17·7(6) | 17·0(4) | 17·6(3) | 19·2(6) | 22·3(3) | 18·2(4) | 17·6(6) | 19·3(7) | 18·4(5) | 18·2(7) | 17·3(5) | 17·8(2) | 18·6(2) | 15·4 | 17·6(2) |
| CaO | 21·0(5) | 21·7(8) | 22·3(5) | 19·7(6) | 21·2(2) | 19·7(7) | 20·3(3) | 20·2(4) | 19·5(7) | 22·0(6) | 16·4(3) | 20·0(2) | 21·4(6) | 24·3(6) | 21·1(4) | 19·0(8) | 20·5(4) | 19·0(2) | 19·6(5) | 18·4 | 21·3(5) |
| Na2O | 2·5(3) | 1·8(4) | 1·6(3) | 2·6(5) | 1·9(1) | 2·2(2) | 2·2(2) | 2·5(2) | 2·6(4) | 0·9(2) | 1·6(1) | 2·1(2) | 1·7(4) | 0·3(3) | 1·6(2) | 2·5(4) | 2·2(2) | 2·0(2) | 1·8(2) | 3·2 | 1·6(2) |
| K2O | 0·2(1) | 0·3(1) | 0·3(0) | 0·5(1) | 0·5(0) | 0·8(1) | 0·5(1) | 0·5(1) | 0·4(1) | 0·6(1) | 0·5(0) | 0·4(0) | 0·4(1) | 0·2(0) | 0·3(0) | 0·4(1) | 0·3(1) | 0·7(3) | 0·6(0) | 0·6 | 0·4(1) |
| ∑ | 99·2(6) | 99·0(4) | 99·0(4) | 98·8(6) | 98·9(3) | 99·1(4) | 100·6(5) | 99·8(7) | 100·8(3) | 100·0(8) | 101·0(6) | 99·9(3) | 99·0(7) | 100·5(8) | 100·0(3) | 100·6(4) | 100·3(4) | 98·9(3) | 99·6(6) | 98·2 | 98·9(3) |
| Si | 1·99(1) | 2·00(1) | 2·00(1) | 1·99(0) | 1·99(0) | 2·00(1) | 2·00(0) | 1·99(0) | 2·00(0) | 2·01(1) | 2·00(1) | 1·99(1) | 2·00(1) | 2·00(1) | 1·99(1) | 2·00(1) | 2·01(0) | 2·01(0) | 2·00(0) | 2·01 | 2·01(0) |
| Al | 0·17(2) | 0·13(3) | 0·12(2) | 0·18(3) | 0·14(1) | 0·16(2) | 0·15(2) | 0·16(2) | 0·16(2) | 0·05(2) | 0·11(0) | 0·14(1) | 0·11(2) | 0·02(2) | 0·12(2) | 0·15(2) | 0·14(1) | 0·14(1) | 0·12(1) | 0·21 | 0·10(1) |
| Mg | 0·87(3) | 0·90(3) | 0·91(3) | 0·88(4) | 0·92(1) | 0·91(2) | 0·93(3) | 0·90(3) | 0·93(2) | 1·02(3) | 1·16(2) | 0·97(2) | 0·95(3) | 1·03(4) | 0·98(3) | 0·96(4) | 0·91(3) | 0·95(1) | 0·99(1) | 0·83 | 0·95(1) |
| Ca | 0·81(2) | 0·84(3) | 0·86(2) | 0·76(3) | 0·82(1) | 0·76(2) | 0·77(1) | 0·77(2) | 0·74(3) | 0·84(2) | 0·61(1) | 0·77(1) | 0·83(2) | 0·93(3) | 0·80(2) | 0·72(3) | 0·78(2) | 0·73(1) | 0·75(2) | 0·71 | 0·82(2) |
| Na | 0·17(2) | 0·12(3) | 0·11(2) | 0·18(3) | 0·13(1) | 0·16(2) | 0·15(2) | 0·17(2) | 0·18(2) | 0·06(1) | 0·11(0) | 0·14(2) | 0·12(3) | 0·02(2) | 0·11(1) | 0·17(3) | 0·15(2) | 0·14(1) | 0·13(1) | 0·22 | 0·11(1) |
| K | 0·01(0) | 0·02(0) | 0·01(0) | 0·02(1) | 0·02(0) | 0·04(1) | 0·02(0) | 0·02(3) | 0·02(2) | 0·03(0) | 0·02(0) | 0·02(0) | 0·02(0) | 0·01(0) | 0·01(1) | 0·02(1) | 0·01(0) | 0·03(0) | 0·03(0) | 0·03 | 0·02(0) |
| ∑ | 4·02(1) | 4·01(1) | 4·01(0) | 4·02(0) | 4·01(0) | 4·01(1) | 4·02(1) | 4·02(5) | 4·02(2) | 4·01(1) | 4·01(1) | 4·02(1) | 4·01(1) | 4·01(1) | 4·02(1) | 4·02(0) | 4·00(1) | 4·00(1) | 4·02(1) | 4·01 | 4·01(0) |
| K/(K+Na) | 0·06(1) | 0·11(2) | 0·09(0) | 0·11(2) | 0·14(1) | 0·19(4) | 0·14(1) | 0·10(1) | 0·09(2) | 0·31(2) | 0·16(1) | 0·10(1) | 0·15(4) | 0·34(2) | 0·11(1) | 0·10(2) | 0·08(1) | 0·18(1) | 0·19(1) | 0·12 | 0·15(1) |
Clinopyroxene formulae recalculated to 6 oxygens.
Average analyses of high-Ca clinopyroxene
| Exp.: | JKW19 | JKW13 | JKW15 | JKW18 | JKW17 | JKW9 | Ma88sB | Ma91B | Ma92B | Ma102M | Ma104M | JKW7 | JKW14 | JKW25 | Ma95sB | Ma94sB | JKW29 | JKW41 | JKW33 | JKW34 | JKW30 |
| Bulk: | per | per | per | per | per | per | per | per | per | per | per | per | per | per | sub | sub | sub | sub | sub | sub | sub |
| P (GPa): | 10 | 10 | 10 | 10 | 11 | 12 | 13 | 14 | 15 | 18 | 13 | 14 | 14 | 15 | 10 | 15 | 14 | 13 | 14 | 14 | 13 |
| T (°C): | 1100 | 1200 | 1300 | 1350 | 1300 | 1300 | 1100 | 1100 | 1100 | 1300 | 1400 | 1300 | 1200 | 900 | 1100 | 1100 | 1100 | 1300 | 1400 | 900 | 1100 |
| No. of analyses: | 8 | 12 | 7 | 8 | 8 | 7 | 10 | 12 | 11 | 4 | 4 | 6 | 11 | 5 | 6 | 5 | 9 | 6 | 7 | 6 | 5 |
| SiO2 | 55·4(4) | 55·4(4) | 55·2(3) | 55·2(5) | 55·0(2) | 55·7(1) | 56·4(2) | 55·8(5) | 56·8(2) | 56·1(7) | 57·5(5) | 55·8(4) | 55·4(6) | 56·0(5) | 55·8(2) | 56·8(3) | 56·8(3) | 56·1(2) | 56·0(3) | 55·7 | 55·6(2) |
| Al2O3 | 3·9(5) | 3·2(6) | 2·8(5) | 4·4(7) | 3·3(3) | 3·8(8) | 3·5(5) | 3·8(4) | 3·9(5) | 1·2(4) | 2·8(1) | 3·2(3) | 2·5(5) | 0·4(3) | 2·8(4) | 3·7(5) | 3·3(3) | 3·3(2) | 2·9(2) | 4·9 | 2·4(3) |
| MgO | 16·2(5) | 16·7(5) | 16·8(5) | 16·4(7) | 17·1(2) | 16·9(4) | 17·7(6) | 17·0(4) | 17·6(3) | 19·2(6) | 22·3(3) | 18·2(4) | 17·6(6) | 19·3(7) | 18·4(5) | 18·2(7) | 17·3(5) | 17·8(2) | 18·6(2) | 15·4 | 17·6(2) |
| CaO | 21·0(5) | 21·7(8) | 22·3(5) | 19·7(6) | 21·2(2) | 19·7(7) | 20·3(3) | 20·2(4) | 19·5(7) | 22·0(6) | 16·4(3) | 20·0(2) | 21·4(6) | 24·3(6) | 21·1(4) | 19·0(8) | 20·5(4) | 19·0(2) | 19·6(5) | 18·4 | 21·3(5) |
| Na2O | 2·5(3) | 1·8(4) | 1·6(3) | 2·6(5) | 1·9(1) | 2·2(2) | 2·2(2) | 2·5(2) | 2·6(4) | 0·9(2) | 1·6(1) | 2·1(2) | 1·7(4) | 0·3(3) | 1·6(2) | 2·5(4) | 2·2(2) | 2·0(2) | 1·8(2) | 3·2 | 1·6(2) |
| K2O | 0·2(1) | 0·3(1) | 0·3(0) | 0·5(1) | 0·5(0) | 0·8(1) | 0·5(1) | 0·5(1) | 0·4(1) | 0·6(1) | 0·5(0) | 0·4(0) | 0·4(1) | 0·2(0) | 0·3(0) | 0·4(1) | 0·3(1) | 0·7(3) | 0·6(0) | 0·6 | 0·4(1) |
| ∑ | 99·2(6) | 99·0(4) | 99·0(4) | 98·8(6) | 98·9(3) | 99·1(4) | 100·6(5) | 99·8(7) | 100·8(3) | 100·0(8) | 101·0(6) | 99·9(3) | 99·0(7) | 100·5(8) | 100·0(3) | 100·6(4) | 100·3(4) | 98·9(3) | 99·6(6) | 98·2 | 98·9(3) |
| Si | 1·99(1) | 2·00(1) | 2·00(1) | 1·99(0) | 1·99(0) | 2·00(1) | 2·00(0) | 1·99(0) | 2·00(0) | 2·01(1) | 2·00(1) | 1·99(1) | 2·00(1) | 2·00(1) | 1·99(1) | 2·00(1) | 2·01(0) | 2·01(0) | 2·00(0) | 2·01 | 2·01(0) |
| Al | 0·17(2) | 0·13(3) | 0·12(2) | 0·18(3) | 0·14(1) | 0·16(2) | 0·15(2) | 0·16(2) | 0·16(2) | 0·05(2) | 0·11(0) | 0·14(1) | 0·11(2) | 0·02(2) | 0·12(2) | 0·15(2) | 0·14(1) | 0·14(1) | 0·12(1) | 0·21 | 0·10(1) |
| Mg | 0·87(3) | 0·90(3) | 0·91(3) | 0·88(4) | 0·92(1) | 0·91(2) | 0·93(3) | 0·90(3) | 0·93(2) | 1·02(3) | 1·16(2) | 0·97(2) | 0·95(3) | 1·03(4) | 0·98(3) | 0·96(4) | 0·91(3) | 0·95(1) | 0·99(1) | 0·83 | 0·95(1) |
| Ca | 0·81(2) | 0·84(3) | 0·86(2) | 0·76(3) | 0·82(1) | 0·76(2) | 0·77(1) | 0·77(2) | 0·74(3) | 0·84(2) | 0·61(1) | 0·77(1) | 0·83(2) | 0·93(3) | 0·80(2) | 0·72(3) | 0·78(2) | 0·73(1) | 0·75(2) | 0·71 | 0·82(2) |
| Na | 0·17(2) | 0·12(3) | 0·11(2) | 0·18(3) | 0·13(1) | 0·16(2) | 0·15(2) | 0·17(2) | 0·18(2) | 0·06(1) | 0·11(0) | 0·14(2) | 0·12(3) | 0·02(2) | 0·11(1) | 0·17(3) | 0·15(2) | 0·14(1) | 0·13(1) | 0·22 | 0·11(1) |
| K | 0·01(0) | 0·02(0) | 0·01(0) | 0·02(1) | 0·02(0) | 0·04(1) | 0·02(0) | 0·02(3) | 0·02(2) | 0·03(0) | 0·02(0) | 0·02(0) | 0·02(0) | 0·01(0) | 0·01(1) | 0·02(1) | 0·01(0) | 0·03(0) | 0·03(0) | 0·03 | 0·02(0) |
| ∑ | 4·02(1) | 4·01(1) | 4·01(0) | 4·02(0) | 4·01(0) | 4·01(1) | 4·02(1) | 4·02(5) | 4·02(2) | 4·01(1) | 4·01(1) | 4·02(1) | 4·01(1) | 4·01(1) | 4·02(1) | 4·02(0) | 4·00(1) | 4·00(1) | 4·02(1) | 4·01 | 4·01(0) |
| K/(K+Na) | 0·06(1) | 0·11(2) | 0·09(0) | 0·11(2) | 0·14(1) | 0·19(4) | 0·14(1) | 0·10(1) | 0·09(2) | 0·31(2) | 0·16(1) | 0·10(1) | 0·15(4) | 0·34(2) | 0·11(1) | 0·10(2) | 0·08(1) | 0·18(1) | 0·19(1) | 0·12 | 0·15(1) |
| Exp.: | JKW19 | JKW13 | JKW15 | JKW18 | JKW17 | JKW9 | Ma88sB | Ma91B | Ma92B | Ma102M | Ma104M | JKW7 | JKW14 | JKW25 | Ma95sB | Ma94sB | JKW29 | JKW41 | JKW33 | JKW34 | JKW30 |
| Bulk: | per | per | per | per | per | per | per | per | per | per | per | per | per | per | sub | sub | sub | sub | sub | sub | sub |
| P (GPa): | 10 | 10 | 10 | 10 | 11 | 12 | 13 | 14 | 15 | 18 | 13 | 14 | 14 | 15 | 10 | 15 | 14 | 13 | 14 | 14 | 13 |
| T (°C): | 1100 | 1200 | 1300 | 1350 | 1300 | 1300 | 1100 | 1100 | 1100 | 1300 | 1400 | 1300 | 1200 | 900 | 1100 | 1100 | 1100 | 1300 | 1400 | 900 | 1100 |
| No. of analyses: | 8 | 12 | 7 | 8 | 8 | 7 | 10 | 12 | 11 | 4 | 4 | 6 | 11 | 5 | 6 | 5 | 9 | 6 | 7 | 6 | 5 |
| SiO2 | 55·4(4) | 55·4(4) | 55·2(3) | 55·2(5) | 55·0(2) | 55·7(1) | 56·4(2) | 55·8(5) | 56·8(2) | 56·1(7) | 57·5(5) | 55·8(4) | 55·4(6) | 56·0(5) | 55·8(2) | 56·8(3) | 56·8(3) | 56·1(2) | 56·0(3) | 55·7 | 55·6(2) |
| Al2O3 | 3·9(5) | 3·2(6) | 2·8(5) | 4·4(7) | 3·3(3) | 3·8(8) | 3·5(5) | 3·8(4) | 3·9(5) | 1·2(4) | 2·8(1) | 3·2(3) | 2·5(5) | 0·4(3) | 2·8(4) | 3·7(5) | 3·3(3) | 3·3(2) | 2·9(2) | 4·9 | 2·4(3) |
| MgO | 16·2(5) | 16·7(5) | 16·8(5) | 16·4(7) | 17·1(2) | 16·9(4) | 17·7(6) | 17·0(4) | 17·6(3) | 19·2(6) | 22·3(3) | 18·2(4) | 17·6(6) | 19·3(7) | 18·4(5) | 18·2(7) | 17·3(5) | 17·8(2) | 18·6(2) | 15·4 | 17·6(2) |
| CaO | 21·0(5) | 21·7(8) | 22·3(5) | 19·7(6) | 21·2(2) | 19·7(7) | 20·3(3) | 20·2(4) | 19·5(7) | 22·0(6) | 16·4(3) | 20·0(2) | 21·4(6) | 24·3(6) | 21·1(4) | 19·0(8) | 20·5(4) | 19·0(2) | 19·6(5) | 18·4 | 21·3(5) |
| Na2O | 2·5(3) | 1·8(4) | 1·6(3) | 2·6(5) | 1·9(1) | 2·2(2) | 2·2(2) | 2·5(2) | 2·6(4) | 0·9(2) | 1·6(1) | 2·1(2) | 1·7(4) | 0·3(3) | 1·6(2) | 2·5(4) | 2·2(2) | 2·0(2) | 1·8(2) | 3·2 | 1·6(2) |
| K2O | 0·2(1) | 0·3(1) | 0·3(0) | 0·5(1) | 0·5(0) | 0·8(1) | 0·5(1) | 0·5(1) | 0·4(1) | 0·6(1) | 0·5(0) | 0·4(0) | 0·4(1) | 0·2(0) | 0·3(0) | 0·4(1) | 0·3(1) | 0·7(3) | 0·6(0) | 0·6 | 0·4(1) |
| ∑ | 99·2(6) | 99·0(4) | 99·0(4) | 98·8(6) | 98·9(3) | 99·1(4) | 100·6(5) | 99·8(7) | 100·8(3) | 100·0(8) | 101·0(6) | 99·9(3) | 99·0(7) | 100·5(8) | 100·0(3) | 100·6(4) | 100·3(4) | 98·9(3) | 99·6(6) | 98·2 | 98·9(3) |
| Si | 1·99(1) | 2·00(1) | 2·00(1) | 1·99(0) | 1·99(0) | 2·00(1) | 2·00(0) | 1·99(0) | 2·00(0) | 2·01(1) | 2·00(1) | 1·99(1) | 2·00(1) | 2·00(1) | 1·99(1) | 2·00(1) | 2·01(0) | 2·01(0) | 2·00(0) | 2·01 | 2·01(0) |
| Al | 0·17(2) | 0·13(3) | 0·12(2) | 0·18(3) | 0·14(1) | 0·16(2) | 0·15(2) | 0·16(2) | 0·16(2) | 0·05(2) | 0·11(0) | 0·14(1) | 0·11(2) | 0·02(2) | 0·12(2) | 0·15(2) | 0·14(1) | 0·14(1) | 0·12(1) | 0·21 | 0·10(1) |
| Mg | 0·87(3) | 0·90(3) | 0·91(3) | 0·88(4) | 0·92(1) | 0·91(2) | 0·93(3) | 0·90(3) | 0·93(2) | 1·02(3) | 1·16(2) | 0·97(2) | 0·95(3) | 1·03(4) | 0·98(3) | 0·96(4) | 0·91(3) | 0·95(1) | 0·99(1) | 0·83 | 0·95(1) |
| Ca | 0·81(2) | 0·84(3) | 0·86(2) | 0·76(3) | 0·82(1) | 0·76(2) | 0·77(1) | 0·77(2) | 0·74(3) | 0·84(2) | 0·61(1) | 0·77(1) | 0·83(2) | 0·93(3) | 0·80(2) | 0·72(3) | 0·78(2) | 0·73(1) | 0·75(2) | 0·71 | 0·82(2) |
| Na | 0·17(2) | 0·12(3) | 0·11(2) | 0·18(3) | 0·13(1) | 0·16(2) | 0·15(2) | 0·17(2) | 0·18(2) | 0·06(1) | 0·11(0) | 0·14(2) | 0·12(3) | 0·02(2) | 0·11(1) | 0·17(3) | 0·15(2) | 0·14(1) | 0·13(1) | 0·22 | 0·11(1) |
| K | 0·01(0) | 0·02(0) | 0·01(0) | 0·02(1) | 0·02(0) | 0·04(1) | 0·02(0) | 0·02(3) | 0·02(2) | 0·03(0) | 0·02(0) | 0·02(0) | 0·02(0) | 0·01(0) | 0·01(1) | 0·02(1) | 0·01(0) | 0·03(0) | 0·03(0) | 0·03 | 0·02(0) |
| ∑ | 4·02(1) | 4·01(1) | 4·01(0) | 4·02(0) | 4·01(0) | 4·01(1) | 4·02(1) | 4·02(5) | 4·02(2) | 4·01(1) | 4·01(1) | 4·02(1) | 4·01(1) | 4·01(1) | 4·02(1) | 4·02(0) | 4·00(1) | 4·00(1) | 4·02(1) | 4·01 | 4·01(0) |
| K/(K+Na) | 0·06(1) | 0·11(2) | 0·09(0) | 0·11(2) | 0·14(1) | 0·19(4) | 0·14(1) | 0·10(1) | 0·09(2) | 0·31(2) | 0·16(1) | 0·10(1) | 0·15(4) | 0·34(2) | 0·11(1) | 0·10(2) | 0·08(1) | 0·18(1) | 0·19(1) | 0·12 | 0·15(1) |
Clinopyroxene formulae recalculated to 6 oxygens.
K-hollandite and Ca-perovskite
K-hollandite is close to stoichiometric KAlSi3O8 with K p.f.u. of 0·97–0·99 and small amounts of Mg and Ca (Table 8). Ca-perovskite is close to CaSiO3 with negligible MgSiO3 component. All analyzed Ca-perovskites contain 0·3–5·5 wt % Al2O3, 0–0·4 wt % Na2O and 0·4–1·0 wt % K2O (Table 8). In the absence of Fe3+, possible mechanisms for incorporation of Al into the Ca-perovskite structure are [XII]Al[VI]Al[XII]Ca−1[VI]Si−1 (Andrault et al., 1998) or [XII]Al[XII]Na[XII]Ca−2 (Kesson et al., 1995). The small number of analyses and the relatively large scatter of the data do not allow us to assess whether or not K can enter the perovskite structure.
Average and representative analyses of Ca-perovskite and K-hollandite
| Exp.: | JKW16 | JKW64 | JKW54 | JKW61 | |||
| Bulk: | per | sub | sub | sub | |||
| P (GPa): | 20·0 | 20·0 | 23·0 | 23·0 | |||
| T (°C): | 1300 | 1600 | 1700 | 1500 | |||
| Phase: | Ca-perov | K-holl | Ca-perov | K-holl | Ca-perov | K-holl | Ca-perov |
| No. of analyses: | 3 | 4 | 4 | 6 | |||
| SiO2 | 50·6 | 66·5(7) | 42·3 | 64·9 | 46·0(8) | 64·5(3) | 45·6(9) |
| Al2O3 | 0·3 | 17·4(4) | 4·6 | 17·2 | 2·1(2) | 18·0(3) | 1·8(2) |
| MgO | 0·3 | 0·8(4) | 0·5 | 0·7 | 0·3(1) | 0·5(5) | 0·2(1) |
| CaO | 46·5 | 0·2(1) | 35·5 | 0·1 | 42·9(9) | 0·1(1) | 44·6(1) |
| Na2O | <0·05 | 0·1(1) | 0·5 | 0·1 | 0·4(1) | 0·1(0) | 0·3(1) |
| K2O | 1·0 | 16·7(1) | 0·4 | 16·6 | 0·4(2) | 16·7(1) | 0·5(3) |
| ∑ | 99·0 | 101·6(5) | 83·8 | 99·5 | 92·0(1) | 99·9(3) | 92·8(1) |
| Si | 0·99 | 3·02(1) | 0·96 | 3·02 | 0·97(1) | 2·99(1) | 0·96(0) |
| Al | 0·01 | 0·93(2) | 0·12 | 0·94 | 0·05(1) | 0·98(1) | 0·05(1) |
| Mg | 0·01 | 0·05(3) | 0·02 | 0·05 | 0·01(0) | 0·03(4) | 0·01(0) |
| Ca | 0·98 | 0·01(1) | 0·86 | 0·01 | 0·97(1) | 0·01(1) | 1·00(1) |
| Na | — | 0·01(1) | 0·02 | 0·01 | 0·02(1) | 0·01(0) | 0·01(1) |
| K | 0·03 | 0·97(1) | 0·01 | 0·99 | 0·01(1) | 0·99(1) | 0·01(1) |
| ∑ | 2·02 | 5·00(2) | 2·00 | 5·01 | 2·02(6) | 5·02(2) | 2·03(1) |
| Exp.: | JKW16 | JKW64 | JKW54 | JKW61 | |||
| Bulk: | per | sub | sub | sub | |||
| P (GPa): | 20·0 | 20·0 | 23·0 | 23·0 | |||
| T (°C): | 1300 | 1600 | 1700 | 1500 | |||
| Phase: | Ca-perov | K-holl | Ca-perov | K-holl | Ca-perov | K-holl | Ca-perov |
| No. of analyses: | 3 | 4 | 4 | 6 | |||
| SiO2 | 50·6 | 66·5(7) | 42·3 | 64·9 | 46·0(8) | 64·5(3) | 45·6(9) |
| Al2O3 | 0·3 | 17·4(4) | 4·6 | 17·2 | 2·1(2) | 18·0(3) | 1·8(2) |
| MgO | 0·3 | 0·8(4) | 0·5 | 0·7 | 0·3(1) | 0·5(5) | 0·2(1) |
| CaO | 46·5 | 0·2(1) | 35·5 | 0·1 | 42·9(9) | 0·1(1) | 44·6(1) |
| Na2O | <0·05 | 0·1(1) | 0·5 | 0·1 | 0·4(1) | 0·1(0) | 0·3(1) |
| K2O | 1·0 | 16·7(1) | 0·4 | 16·6 | 0·4(2) | 16·7(1) | 0·5(3) |
| ∑ | 99·0 | 101·6(5) | 83·8 | 99·5 | 92·0(1) | 99·9(3) | 92·8(1) |
| Si | 0·99 | 3·02(1) | 0·96 | 3·02 | 0·97(1) | 2·99(1) | 0·96(0) |
| Al | 0·01 | 0·93(2) | 0·12 | 0·94 | 0·05(1) | 0·98(1) | 0·05(1) |
| Mg | 0·01 | 0·05(3) | 0·02 | 0·05 | 0·01(0) | 0·03(4) | 0·01(0) |
| Ca | 0·98 | 0·01(1) | 0·86 | 0·01 | 0·97(1) | 0·01(1) | 1·00(1) |
| Na | — | 0·01(1) | 0·02 | 0·01 | 0·02(1) | 0·01(0) | 0·01(1) |
| K | 0·03 | 0·97(1) | 0·01 | 0·99 | 0·01(1) | 0·99(1) | 0·01(1) |
| ∑ | 2·02 | 5·00(2) | 2·00 | 5·01 | 2·02(6) | 5·02(2) | 2·03(1) |
K-hollandite and Ca-perovskite formulae recalculated to 2 and 8 oxygens, respectively.
Average and representative analyses of Ca-perovskite and K-hollandite
| Exp.: | JKW16 | JKW64 | JKW54 | JKW61 | |||
| Bulk: | per | sub | sub | sub | |||
| P (GPa): | 20·0 | 20·0 | 23·0 | 23·0 | |||
| T (°C): | 1300 | 1600 | 1700 | 1500 | |||
| Phase: | Ca-perov | K-holl | Ca-perov | K-holl | Ca-perov | K-holl | Ca-perov |
| No. of analyses: | 3 | 4 | 4 | 6 | |||
| SiO2 | 50·6 | 66·5(7) | 42·3 | 64·9 | 46·0(8) | 64·5(3) | 45·6(9) |
| Al2O3 | 0·3 | 17·4(4) | 4·6 | 17·2 | 2·1(2) | 18·0(3) | 1·8(2) |
| MgO | 0·3 | 0·8(4) | 0·5 | 0·7 | 0·3(1) | 0·5(5) | 0·2(1) |
| CaO | 46·5 | 0·2(1) | 35·5 | 0·1 | 42·9(9) | 0·1(1) | 44·6(1) |
| Na2O | <0·05 | 0·1(1) | 0·5 | 0·1 | 0·4(1) | 0·1(0) | 0·3(1) |
| K2O | 1·0 | 16·7(1) | 0·4 | 16·6 | 0·4(2) | 16·7(1) | 0·5(3) |
| ∑ | 99·0 | 101·6(5) | 83·8 | 99·5 | 92·0(1) | 99·9(3) | 92·8(1) |
| Si | 0·99 | 3·02(1) | 0·96 | 3·02 | 0·97(1) | 2·99(1) | 0·96(0) |
| Al | 0·01 | 0·93(2) | 0·12 | 0·94 | 0·05(1) | 0·98(1) | 0·05(1) |
| Mg | 0·01 | 0·05(3) | 0·02 | 0·05 | 0·01(0) | 0·03(4) | 0·01(0) |
| Ca | 0·98 | 0·01(1) | 0·86 | 0·01 | 0·97(1) | 0·01(1) | 1·00(1) |
| Na | — | 0·01(1) | 0·02 | 0·01 | 0·02(1) | 0·01(0) | 0·01(1) |
| K | 0·03 | 0·97(1) | 0·01 | 0·99 | 0·01(1) | 0·99(1) | 0·01(1) |
| ∑ | 2·02 | 5·00(2) | 2·00 | 5·01 | 2·02(6) | 5·02(2) | 2·03(1) |
| Exp.: | JKW16 | JKW64 | JKW54 | JKW61 | |||
| Bulk: | per | sub | sub | sub | |||
| P (GPa): | 20·0 | 20·0 | 23·0 | 23·0 | |||
| T (°C): | 1300 | 1600 | 1700 | 1500 | |||
| Phase: | Ca-perov | K-holl | Ca-perov | K-holl | Ca-perov | K-holl | Ca-perov |
| No. of analyses: | 3 | 4 | 4 | 6 | |||
| SiO2 | 50·6 | 66·5(7) | 42·3 | 64·9 | 46·0(8) | 64·5(3) | 45·6(9) |
| Al2O3 | 0·3 | 17·4(4) | 4·6 | 17·2 | 2·1(2) | 18·0(3) | 1·8(2) |
| MgO | 0·3 | 0·8(4) | 0·5 | 0·7 | 0·3(1) | 0·5(5) | 0·2(1) |
| CaO | 46·5 | 0·2(1) | 35·5 | 0·1 | 42·9(9) | 0·1(1) | 44·6(1) |
| Na2O | <0·05 | 0·1(1) | 0·5 | 0·1 | 0·4(1) | 0·1(0) | 0·3(1) |
| K2O | 1·0 | 16·7(1) | 0·4 | 16·6 | 0·4(2) | 16·7(1) | 0·5(3) |
| ∑ | 99·0 | 101·6(5) | 83·8 | 99·5 | 92·0(1) | 99·9(3) | 92·8(1) |
| Si | 0·99 | 3·02(1) | 0·96 | 3·02 | 0·97(1) | 2·99(1) | 0·96(0) |
| Al | 0·01 | 0·93(2) | 0·12 | 0·94 | 0·05(1) | 0·98(1) | 0·05(1) |
| Mg | 0·01 | 0·05(3) | 0·02 | 0·05 | 0·01(0) | 0·03(4) | 0·01(0) |
| Ca | 0·98 | 0·01(1) | 0·86 | 0·01 | 0·97(1) | 0·01(1) | 1·00(1) |
| Na | — | 0·01(1) | 0·02 | 0·01 | 0·02(1) | 0·01(0) | 0·01(1) |
| K | 0·03 | 0·97(1) | 0·01 | 0·99 | 0·01(1) | 0·99(1) | 0·01(1) |
| ∑ | 2·02 | 5·00(2) | 2·00 | 5·01 | 2·02(6) | 5·02(2) | 2·03(1) |
K-hollandite and Ca-perovskite formulae recalculated to 2 and 8 oxygens, respectively.
DISCUSSION
K-amphibole breakdown
In the KLB-1 bulk composition K-amphibole breaks down between 12 and 13 GPa at 1200°C, equivalent to depths of 360–390 km. Within this depth interval, the α to α + β transition occurs for olivine (Fo90) (Katsura & Ito 1989). Because of the high solubility of H2O in Mg2SiO4 (0·1–0·15 wt % in ol at 10–13 GPa; 2·1–2·4 wt % in β-Mg2SiO4 at 14–15 GPa; Young et al., 1993; Kohlstedt et al., 1996), the generation of a free H2O fluid from K-amphibole breakdown is unlikely, as was noted by Inoue et al. (1998).
Stability of hydrous potassic phases along the ACMA
Experiments of this study combined with those by Konzett et al. (1997) indicate that in peralkaline bulk compositions hydrous potassic phases break down at temperatures slightly below an ACMA defined by 17·9 GPa and 1475°C for the β → γ transition in Mg2SiO4 and 23·2 GPa and 1530°C for the γ → perovskite + magnesiowüstite transition (see Agee, 1998). Because the breakdown of HPPs in the peralkaline KNCMASH system occurs ∼50–100°C below the ACMA, only large amounts of F can stabilize HPPs along the ACMA (Foley, 1991). Otherwise, a hydrous melt will carry most of the K under P–T conditions of an ACMA, and the K content of the solid residue will reflect partitioning between high-Ca clinopyroxene and melt. In run Ma104M at 13 GPa and 1400°C the quench has 6·6 ± 0·5 wt % K2O (n = 3) compared with 0·45 ± 0·02 wt % K2O in high-Ca clinopyroxene with a resulting DKhiCapx–liquid of 0·07 (because of the possibility that H2O-soluble K-rich material was lost from the quench as a result of H2O saturation, the D value has to be considered a maximum value). This value is consistent with DKhiCapx–liquid of 0·02–0·15 obtained by Luth (1997) and Tsuruta & Takahashi (1998) from experiments in a simplified phlogopite + diopside and a dry natural alkali basalt system. In our experiments the K content of high-Ca clinopyroxene is <0·7 wt % K2O, even at P = 18 GPa. This is consistent with the hypothesis of Luth (1997) that in a peridotitic bulk composition high-Ca clinopyroxene cannot accommodate significant K in the presence of an HPP (either solid or melt). The formation of K-rich (up to 1·7 wt % K2O; Harlow & Veblen, 1991) high-Ca clinopyroxene found as diamond inclusions probably requires the presence of C- and K-rich (carbonatitic) melts as suggested by Harlow (1997). In metabasaltic compositions, however, omphacitic high-Ca clinopyroxene coexisting with phengite can accommodate up to 1·1 wt % K2O (Schmidt, 1996).
In the subalkaline bulk composition, the stability of HPPs extends to temperatures significantly higher than those of an ACMA, above the solidus of H2O-bearing peridotite (Fig. 3; Kawamoto et al., 1996; Kawamoto & Holloway, 1997). Luth (1997) reported the assemblage phase X + olivine + garnet + clinopyroxene + liquid at 11 GPa and 1600°C, which suggests a potential supersolidus stability of phase X. In our experiments, phase X coexists with small amounts of quench over a temperature interval of at least 200°C. The textures do not demonstrate whether the quench formed by crystallization from a hydrous melt or a solute-rich fluid, and changes in XMg of the phases as a result of the presence of melt (Kawamoto et al., 1996) could not be used in the KNCMASH system. Nevertheless, our data indicate that phase X is stable under the P–T conditions of convecting mantle, and that it coexists with hydrous peridotite melt over a wide pressure range. Thus, a supersolidus stability of phase X should control the large ion lithophile element (LILE) budget of coexisting partial melts. Although no trace element partition coefficients exist for phase X, it should, like other HPPs, store and retain large ions such as Cs, Rb, Ba or Pb in the large potassium lattice position (Kramers et al., 1983; Rosenbaum, 1993; Irifune et al., 1994; Ionov et al., 1997).
Potassium recycling in subduction zones
Experimental studies suggest that within subducted oceanic crust [mid-ocean ridge basalt (MORB), andesites, graywackes] the major subsolidus potassium carrier is phengite, which is stable to 10 GPa and breaks down to form K-hollandite + K-rich fluid at P > 10 GPa (Domanik & Holloway, 1996; Schmidt, 1996). K-feldspar would survive initial stages of subduction only under fluid-absent conditions but could react to form K-cymrite (hydrous hexasanidine KAlSi3O8.nH2O) under fluid-present conditions and remain stable in the hydrated form to 8 GPa (George E. Harlow, personal communication, 1999). Thus, potassium and water transport are likely to be coupled to 300 km depths in Al-rich metasedimentary bulk compositions. Dehydration and/or melting reactions within the slab involving HPPs such as continuous phengite dehydration, K-MORB melting or Ca-amphibole and phlogopite breakdown at <3 GPa (Domanik & Holloway, 1996; Schmidt, 1996) can provide K and H2O and stabilize HPPs within the peridotitic mantle wedge. With increasing P, the succession is phlogopite (± Ca-amphibole) → K-richterite → phase X, with final dehydration of phase X to K-hollandite at P ≥ 20 GPa or 600 km. Decoupling of K and H2O in the mantle wedge therefore will occur at the base of the transition zone, or 300 km deeper than in the subducting slab. Any K transferred to the mantle wedge below 150 km will be lost for recycling by arc magmatism, and dragged down into the lower mantle in K-hollandite.
For most subduction zone geometries (see Davies & Stevenson, 1992; Schmidt & Poli, 1998) the partially molten zone that feeds arc volcanism lies above a region of the mantle wedge in which phlogopite is stable. Xenolithic evidence (e.g. Swanson et al., 1987; Canil & Scarfe, 1988; McGibbon et al., 1988; Ionov & Hofmann, 1995; Ertan & Leeman, 1996) confirms the presence of phlogopite in subarc mantle. Assuming that isotherms parallel the subducting slab, it is difficult to transport phlogopite-bearing peridotite into the melting zone of the wedge (>1200°C at 3 GPa for phlogopite melting; see Wendlandt & Eggler, 1980). Lateral transport of phlogopite through a mechanism such as that proposed by Davies & Stevenson (1992) for amphibole is unsuitable because of the much higher P stability of phlogopite compared with amphibole. On the basis of experimentally derived partition coefficients, LaTourrette et al. (1995) showed that phlogopite in the residue of an arc magma would be inconsistent with typical trace element patterns of arc lavas (e.g. Saunders et al., 1991) because residual phlogopite would strongly retain LILE. Thus, the enrichment in LILE of arc lavas might be due to either complete extraction of phlogopite with the degree of LILE enrichment controlled by the modal amount of phlogopite in the source or by addition of LILE-enriched melts or fluids rising from the slab through channels without pervasive phlogopite formation.
APPENDIX
List of mineral end-members, abbreviations and #formulae used in this study
| Mineral | Abbreviation | Formula |
| K-richterite | Kr | KNaCaMg5Si8O22(OH)2 |
| phase X | pX | KHMg2Si2O7 |
| Na-bearing phase X | NapX | NaHMg2Si2O7 |
| pyrope | py | Mg3Al2Si3O12 |
| grossular | gross | Ca3Al2Si3O12 |
| jadeite | jad | NaAlSi2O6 |
| diopside | di | CaMgSi2O6 |
| clinoenstatite | cen | Mg2Si2O6 |
| phlogopite | phl | |
| pyribole | pyr | |
| high-Ca clinopyroxene | hiCapx | |
| low-Ca clinopyroxene | loCapx | |
| K-hollandite | K-holl | |
| Ca-perovskite | Ca-perov | |
| omphacite | omph | |
| garnet (majoritic) | ga | |
| quenched fluid or melt | Q |
| Mineral | Abbreviation | Formula |
| K-richterite | Kr | KNaCaMg5Si8O22(OH)2 |
| phase X | pX | KHMg2Si2O7 |
| Na-bearing phase X | NapX | NaHMg2Si2O7 |
| pyrope | py | Mg3Al2Si3O12 |
| grossular | gross | Ca3Al2Si3O12 |
| jadeite | jad | NaAlSi2O6 |
| diopside | di | CaMgSi2O6 |
| clinoenstatite | cen | Mg2Si2O6 |
| phlogopite | phl | |
| pyribole | pyr | |
| high-Ca clinopyroxene | hiCapx | |
| low-Ca clinopyroxene | loCapx | |
| K-hollandite | K-holl | |
| Ca-perovskite | Ca-perov | |
| omphacite | omph | |
| garnet (majoritic) | ga | |
| quenched fluid or melt | Q |
List of mineral end-members, abbreviations and #formulae used in this study
| Mineral | Abbreviation | Formula |
| K-richterite | Kr | KNaCaMg5Si8O22(OH)2 |
| phase X | pX | KHMg2Si2O7 |
| Na-bearing phase X | NapX | NaHMg2Si2O7 |
| pyrope | py | Mg3Al2Si3O12 |
| grossular | gross | Ca3Al2Si3O12 |
| jadeite | jad | NaAlSi2O6 |
| diopside | di | CaMgSi2O6 |
| clinoenstatite | cen | Mg2Si2O6 |
| phlogopite | phl | |
| pyribole | pyr | |
| high-Ca clinopyroxene | hiCapx | |
| low-Ca clinopyroxene | loCapx | |
| K-hollandite | K-holl | |
| Ca-perovskite | Ca-perov | |
| omphacite | omph | |
| garnet (majoritic) | ga | |
| quenched fluid or melt | Q |
| Mineral | Abbreviation | Formula |
| K-richterite | Kr | KNaCaMg5Si8O22(OH)2 |
| phase X | pX | KHMg2Si2O7 |
| Na-bearing phase X | NapX | NaHMg2Si2O7 |
| pyrope | py | Mg3Al2Si3O12 |
| grossular | gross | Ca3Al2Si3O12 |
| jadeite | jad | NaAlSi2O6 |
| diopside | di | CaMgSi2O6 |
| clinoenstatite | cen | Mg2Si2O6 |
| phlogopite | phl | |
| pyribole | pyr | |
| high-Ca clinopyroxene | hiCapx | |
| low-Ca clinopyroxene | loCapx | |
| K-hollandite | K-holl | |
| Ca-perovskite | Ca-perov | |
| omphacite | omph | |
| garnet (majoritic) | ga | |
| quenched fluid or melt | Q |
*Corresponding author. Present address: Institut für Mineralogie und Petrographie, Universität Innsbruck, Innrain 52, A-6020 Innsbruck, Austria. Telephone: +43-512-507-5506. Fax: +43-512-507-2926. e-mail: juergen.konzett@uibk.ac.at
Part of the multianvil experiments were performed at the Bayerisches Geoinstitut under the EC ‘Human Capital and Mobility—Access to Large Scale Facilities’ programme (Contract ERBCHGECT940053 to D. C. Rubie). Sincere thanks go to Dave Rubie for providing access to the high-pressure equipment, and especially to Max Schmidt for sacrificing his time and nerves in an effort to protect the equipment from destruction while it was being used by J.K. We would also like to thank Bjørn Mysen for his help with Raman spectroscopy, and Hexiong Yang for helpful comments on an early version of the manuscript. Eiichi Takahashi kindly provided a sample of KLB-1. Reviews by George Harlow, Bob Luth, and Anne Peslier helped to improve the manuscript and are gratefully acknowledged. This work was supported by the Swiss National Science Foundation, the NSF Center for High Pressure Research, and the Carnegie Institution of Washington.
REFERENCES
Agee, C. B. (
Andrault, D., Neuville, D. R., Flank, A.-M. & Wang, Y. (
Basu, A. R. (
Bertka, C. M. & Fei, Y. (
Canil, D. & Scarfe, C. M. (
Davies, J. H. & Stevenson, D. J. (
Dawson, J. B. & Smith, J. V. (
Domanik, K. J. & Holloway, J. R. (
Ertan, I. E. & Leeman, W. P. (
Esperança, S. & Holloway, J. R. (
Finger, L. W., Yang, H., Konzett, J. & Fei, Y. (
Foley, S. (
Foley, S. F. (
Foley, S. F., Jenner, G. A., Konzett, J. & Sweeney, R. J. (
Frost, D. J. & Fei, Y. (
Gasparik, T. (
Gilbert, M. C. & Briggs, D. F. (
Harlow, G. E. (
Harlow, G. E. & Veblen, D. R. (
Inoue, T., Irifune, T., Yurimoto, H. & Miyagi, I. (
Inoue, T., Yurimoto, H. & Kudoh, Y. (
Inoue, T., Irifune, T., Yurimoto, H. & Miyagi, I. (
Ionov, D. A. & Hofmann, A. W. (
Ionov, D. A., Griffin, W. L. & O’Reilly, S. O. (
Irifune, T., Ringwood, A. E. & Hibberson, W. O. (
Ito, E. & Takahashi, E. (
Kanzaki, M. (
Katsura, T. & Ito, E. (
Kawamoto, T. & Holloway, J. R. (
Kawamoto, T., Hervig, R. L. & Holloway, J. R. (
Kesson, S. E., Fitz Gerald, J. D., Shelley, J. M. G. & Withers, R. L. (
Kohlstedt, D. L., Keppler, H. & Rubie, D. C. (
Konzett, J. & Fei, Y. (
Konzett, J. & Ulmer, P. (
Konzett, J. & Yang, H. (
Konzett, J., Sweeney, R. J., Thompson, A. B. & Ulmer, P. (
Kramers, J. D., Roddick, J. C. M. & Dawson, J. B. (
Kudoh, Y., Finger, L. W., Hazen, R. M., Prewitt, C. T., Kanzaki, M. & Veblen, D. R. (
Kudoh, Y., Inoue, T. & Arashi, H. (
LaTourrette, T., Hervig, R. L. & Holloway, J. R. (
Leake, B. E., Woolley, A. R., Arps, C. E. S., Birch, W. D., Gilbert, M. C., Grice, J. D., Hawthorne, F. C., Kato, A., Kisch, H. J., Krivovichev, V. G., Linthout, K., Laird, J., Mandarino, J. A., Maresch, W. V., Nickel, E. H., Rock, N. M. S., Schumacher, J. C., Smith, D. C., Stephenson, N. C. N., Ungaretti, L., Whittaker, E. J. W. & Youzhi, G. (
Lesher, C. E. & Walker, D. (
Libau, F. (
Luth, R. W. (
Luth, R. W. (
Massonne, H.-J. (
Massonne, H.-J. & Schreyer, W. (
McGibbon, F. M., Hawkesworth, C. J. & Menzies, M. A. (
Mengel, K. & Green, D. H. (
Morishima, H., Kato, T., Suto, M., Ohtani, E., Urakawa, S., Utsumi, W., Shimomura, O. & Kikegawa, T. (
Oguri, K., Funamori, N., Sakai, F., Kondo, T., Uchida, T. & Yagi, T. (
Ringwood, A. E. (
Ringwood, A. E. & Major, A. (
Rogers, N. W. (
Rosenbaum, J. M. (
Rubie, D. C., Karato, S., Yan, H. & O’Neill, H. St C. (
Sato, K. (
Sato, K., Katsura, T. & Ito, E. (
Saunders, A. D., Norry, M. J. & Tarney, J. (
Schmidt, M. W. (
Schmidt, M. W. (
Schmidt, M. W. & Poli, S. (
Seifert, F. & Schreyer, W. (
Sudo, A. & Tatsumi, Y. (
Swanson, S. E., Kay, S. M., Brearley, M. & Scarfe, C. M. (
Takahashi, E. (
Taylor, W. R., Tompkins, L. A. & Haggerty, S. F. (
Thompson, J. B. (
Trønnes, R. G. (
Trønnes, R. G., Takahashi, E. & Scarfe, C. M. (
Tsuruta, K. & Takahashi, E. (
Veblen, D. R. (
Wendlandt, R. F. & Eggler, D. H. (
Wilkinson, J. F. G. & Le Maitre, R. W. (
Yang, H., Konzett, J., Prewitt, C. T. & Fei, Y. (