-
PDF
- Split View
-
Views
-
Cite
Cite
CAROLE CORDIER, JEAN-PHILIPPE CLÉMENT, MARTIAL CAROFF, CHRISTOPHE HÉMOND, SYLVAIN BLAIS, JOSEPH COTTEN, CLAIRE BOLLINGER, PATRICK LAUNEAU, GÉRARD GUILLE, Petrogenesis of Coarse-grained Intrusives from Tahiti Nui and Raiatea (Society Islands, French Polynesia), Journal of Petrology, Volume 46, Issue 11, November 2005, Pages 2281–2312, https://doi.org/10.1093/petrology/egi055
Close - Share Icon Share
Abstract
This study is based on a set of coarse-grained igneous rocks collected from two zoned plutons located in the central part of Tahiti Nui and Raiatea. The Ahititera pluton (central depression of Tahiti Nui) comprises a great diversity of rocks, ranging from ultrabasic to felsic in composition. It shows a concentric zonation with nepheline-free rocks in its periphery and nepheline-bearing rocks in its central part. The Faaroa pluton (central depression of Raiatea) is entirely mafic and includes only gabbros and theralites. The two plutons have variable Nd–Sr isotopic signatures, especially the Ahititera rocks, which are subdivided into three groups based on their mineralogy, geochemistry and isotope composition. The isotopic variability probably reflects local heterogeneities in the Society mantle plume. Petrographic and isotopic data have been used to define two magmatic suites in Ahititera, identifiable from their degree of Si undersaturation. The evolution of the mildly Si-undersaturated suite is controlled by simple fractional crystallization, whereas the strongly Si-undersaturated suite requires additional H2O influx. The third isotopic group includes only theralites. The rare earth element (REE) compositions of the mafic rocks from both plutons do not correlate with their isotopic signature. The REE patterns of the most Si-undersaturated rocks are systematically characterized by steeper slopes. Such features are also observed in lavas from seamounts located within the present-day hotspot area. It appears that REE concentrations in Society lavas and intrusives are probably mainly governed by variable degrees of partial melting of a garnet-free mantle source and are independent of their isotopic signature.
INTRODUCTION
Most studies of ocean island magmatism have focused on the petrology and geochemistry of lavas. These data have allowed discussion of the chemical characteristics of the mantle source (e.g. Gautier et al., 1990; Späth et al., 1996), the influence of crustal assimilation (e.g. Bohrson & Reid, 1995) and the characteristics of the petrogenetic processes (e.g. Nekvasil et al., 2000; Thompson et al., 2001). Over the last 20 years, many such studies have focused on French Polynesia; the most interesting results have highlighted trace element variabilities and isotopic heterogeneities at various scales: between archipelagoes (e.g. Hémond et al., 1994), individual edifices (e.g. Cheng et al., 1993) or between different volcanic units within a single island (e.g. Ielsch et al., 1998).
Coarse-grained plutonic rocks are relatively uncommon in oceanic intraplate volcanic domains, except as xenoliths (Fodor et al., 1993; Hoover & Fodor, 1997) or as fragments within pyroclastic breccias (e.g. Freundt-Malecha et al., 2001). When intrusive rocks crop out in such contexts, they are generally small bodies, 10–100 m in diameter (Staudigel & Schmincke, 1984), or ‘coherent intrusion complexes’, formed by networks of dykes and/or sheets (Walker, 1992; Schirnick et al., 1999). Exposures of large zoned plutonic bodies such as those occurring in continental settings (e.g. stocks, ring complexes, large sills) are especially rare in ocean islands (Kerguelen: Giret et al., 1988). Such intrusions are generally accessible only through drill holes (La Réunion: Rançon et al., 1989). In addition, although the petrographic types recognized within the plutonic rocks are highly variable, there are only few trace element and isotope data available in literature.
Coarse-grained rocks have been sampled in most of the Society Islands, either as xenolithic blocks (Moorea: Le Dez et al., 1998; Huahine: Legendre et al., 2003), or in situ. Two types of intrusions can be recognized. Small bodies less than 500 m in diameter occur at the periphery of the islands of Bora Bora, Maupiti, and Tahiti (heterogeneous complex of Taiarapu Peninsula). Large petrographically zoned plutons, 1–2 km2 in surface area, are exposed in the central part of the calderas of Tahiti Nui and Raiatea (Fig. 1). Each of them is made up of more or less strongly silica-undersaturated alkaline intrusives.
Sketch maps of Tahiti Nui (a) and Raiatea (b) showing the position of the plutonic bodies of Ahititera and Faaroa, respectively, within horseshoe-shaped calderas. Inset: location of Tahiti and Raiatea in the Society Archipelago.
The purpose of this paper is to document the petrogenesis of the two large plutons of the Society Archipelago. We have recognized a wide spectrum of petrographic types in the Raiatea and Tahiti Nui plutonic bodies. Detailed textural study allows identification of cumulus and intercumulus phases and these data are used to correct the bulk-rock chemical compositions. Late- and post-magmatic phases are used to analyse the end of the crystallization process. Based on the large set of major and trace element data available for Tahiti Nui, fractionating assemblages have been modelled for each evolutionary stage of both magmatic suites, and the consequences of fluid input during magmatic differentiation examined. The results highlight the role of high-temperature fluids in the evolution of ocean island magmas. Finally, the strongly contrasted isotopic signatures in the Tahitian plutonic rocks are used to discuss their petrogenesis by variable degrees of partial melting of a heterogeneous mantle source, by a comparison with Society mafic lavas.
GEOLOGICAL CONTEXT
Tahiti and Raiatea in the Society Archipelago
The Society Islands, one of the five linear volcanic chains of French Polynesia, consist of nine major volcanic islands and several atolls and seamounts (Fig. 1 inset). The average ages of the exposed lavas decrease from NW (Maupiti) to SE (Mehetia) (Duncan & McDougall, 1976; Cheng et al., 1993; Blais et al., 2002). The current hotspot zone is located around Mehetia, 50 km SE of Tahiti, as shown by the occurrence of several very young seamounts and by volcano-seismic activity (Binard et al., 1991).
Tahiti (17°30′S, 149°30′W), the largest island of the Society Archipelago, is built on c. 3500 m deep ocean crust. Tahiti is made up of two coalescent eroded volcanoes: Tahiti Nui, 2241 m high and 35 km in diameter, and Taiarapu (Fig. 1a). The subaerial products of Tahiti Nui, dated between 1367 ± 16 ka and 187 ± 3 ka (Le Roy, 1994; Hildenbrand et al., 2004), are mainly mafic and intermediate lavas which become increasingly silica undersaturated with time (basalts, basanites, foidites and tephrites: McBirney & Aoki, 1968; Cheng et al., 1993; Duncan & Fisk, 1994). Differentiated lavas (trachytes and phonolites) are very sparse. The plutonic body crops out inside a ‘horseshoe-shaped’ depression which opens towards the NNE. Debris avalanche deposits led Clément et al. (2002) to interpret this caldera as the scar of a huge gravity landslide. This event may have been caused by a local edifice destabilization as a consequence of the pluton emplacement between 570 and 390 ka.
Raiatea (16°49′S, 151°15′W), the second largest island of the Society chain, lies 220 km NE of Tahiti and reaches 1017 m above sea level. The shield volcano, made up of picrites, basalts, hawaiites and trachytes, was dated between 2·75 and 2·44 Ma (Blais et al., 1997; Guillou et al., 1998). A late fissural trachytic event has generated the post-shield plateau of Temehani. In the southern part of the island, plutonic rocks crop out inside the ‘horseshoe-shaped’ Faaroa depression which opens towards the NE (Fig. 1b). The age of the caldera formation has been estimated at about 2·53 Ma and its origin is ascribed either to a mega-landslide or to a collapse (Blais et al., 1997; Dauteuil et al., 1998; Guillou et al., 1998). The age of the Raiatea pluton is unknown.
This study is based on a set of 35 fresh or very slightly altered rocks, sampled during a field trip to Tahiti and Raiatea (October 1999) supported by CEA (Commissariat à l'Energie Atomique) and BRGM (Bureau de Recherches Géologiques et Minières), complemented with five additional samples previously collected.
Geological setting of the two studied plutons
Tahiti Nui
The Ahititera pluton, discovered by J. D. Dana in 1849, was first sampled by R. Brousse and G. Guille in 1971 (Nitecki-Novotny, 1975), a sample set completed by J.-M. Bardintzeff in 1981 (Bardintzeff et al., 1988). It is exposed over an area of 2·1 km2 in the central part of a depression 8 km in diameter, 759 m at its highest point (Ahititera Mount, Fig. 2a). It has an 2·6 km east–west elongated shape. The plutonic body is circled by three rivers: Maroto, in the north, Vaituoru in the east and Ieifatautau in the south (Fig. 2a). It is partially covered by epiclastic volcanic breccias bearing a few coarse-grained clasts (Clément et al., 2002; Clément & Caroff, 2004). Deterioration of the outcrops between 1971 and 1999, as a result of the proliferation of Miconia calvescens, required us to collect all the samples from the periphery of the pluton, along the river beds. From the 46 samples collected in (or close to) the Ahititera plutonic body during the 1999 field investigations, 26 were selected for this study. We have complemented our sample set with two previously studied rocks (37H and TP6: Nitecki-Novotny, 1975; Bardintzeff et al., 1988). The total sample set (28 samples) includes nine petrographic types. The rocks are found either in place, or as large boulders, or as clasts in the debris avalanche deposits (Table 1, Fig. 2a). A large hydrothermally altered intrusion near the Vaituoru river dam is crosscut by numerous small altered basanitic and tephritic dykes. Two large east–west-trending dykes cut the pluton near the Vaituoru river dam and north of Maroto river, respectively (Fig. 2a).
Set of coarse-grained rocks sampled in Ahititera pluton (Tahiti Nui) and in Faaroa pluton (Raiatea)
| Petrographic types . | Samples . | Outcropping . | Location . | Textural types . | Distinctive features . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ahititera pluton | ||||||||||
| Ultrabasic rocks | ||||||||||
| Olivine-clinopyroxenite | THG-5C | Clast | East of the pluton | Cumulate | ||||||
| THG-10B | In place | Ieifatautau | Cumulate | Lobate contact with THG-10C | ||||||
| Clinopyroxene-hornblendite | THG-2D | Block | Maroto | Cumulate | ||||||
| THG-14 | Block | Vaituoru | Cumulate | |||||||
| THG-7A | Clast | East of the pluton | Cumulate | |||||||
| Nepheline-free rocks = mildly Si-undersaturated rocks | ||||||||||
| Gabbro | THG-1B | Block | Maroto | Non-cumulative | ||||||
| THG-1 Da | Block | Maroto | Non-cumulative | Contains THG-1Db as a small dyke | ||||||
| THG-7B | Clast | East of the pluton | Non-cumulative | |||||||
| THG-10C | In place | Ieifatautau | Non-cumulative | Lobate contact with THG-10B | ||||||
| THG-10E | Block | Ieifatautau | Moderately cumulative | Layered | ||||||
| Monzonite | 37H | Block | Vaituoru | Non-cumulative | Sampled by R. Brousse and G. Guille in 1971 | |||||
| TP6 | Block | Papenoo | Non-cumulative | Sampled by J.-M. Bardintzeff in 1981 | ||||||
| Alkali syenite | THG-9B | In place | Near the Vaituoru dam | Non-cumulative | Hydrothermalized sample | |||||
| Nepheline-bearing rocks = strongly Si-undersaturated rocks | ||||||||||
| Theralite | THG-1A | Block | Maroto | Moderately cumulative | ||||||
| THG-2A | Block | Maroto | Moderately cumulative | |||||||
| THG-2B | Block | Maroto | Non-cumulative | |||||||
| THG-9E2 | Block | Vaituoru | Non-cumulative | Layered | ||||||
| THG-13A | Clast | East of the pluton | Non-cumulative | |||||||
| Essexite | THG-1E | Block | Maroto | Non-cumulative | ||||||
| THG-2C | Block | Maroto | Non-cumulative | |||||||
| THG-3A | Block | South of Maroto | Moderately cumulative | Contains THG-3B as a lobate vein | ||||||
| THG-9E1 | In place | Near the Vaituoru dam | Non-cumulative | |||||||
| THG-11A | Block | Ieifatautau | Moderately cumulative | |||||||
| Nepheline-monzosyenite | THG-4 | In place | South of Maroto | Non-cumulative | ||||||
| THG-6A | Clast | East of the pluton | Non-cumulative | |||||||
| Nepheline-syenite | THG-1Db | Block | Maroto | Non-cumulative | Small dyke in THG-1 Da | |||||
| THG-3B | Block | South of Maroto | Non-cumulative | Lobate vein in THG-3A | ||||||
| THG-19 | Clast | East of the pluton | Non-cumulative | |||||||
| Faaroa pluton | ||||||||||
| Nepheline-free rocks = mildly Si-undersaturated rocks | ||||||||||
| Gabbro | RIG-3B | In place | Apoomau: southern tributary | Non-cumulative | ||||||
| RI-28 | In place | Apoomau: southern tributary | Non-cumulative | Sampled by S. Blais in 1994 | ||||||
| Nepheline-bearing rocks = strongly Si-undersaturated rocks | ||||||||||
| Theralite | RIG-1B | In place | East of the crossroad | Non-cumulative | ||||||
| RIG-1E | In place | East of the crossroad | Non-cumulative | |||||||
| RIG-2A | In place | West of the crossroad | Non-cumulative | |||||||
| RIG-2C | In place | West of the crossroad | Non-cumulative | |||||||
| RIG-2D | In place | West of the crossroad | Non-cumulative | |||||||
| RIG-4A | In place | Apoomau: northern tributary | Non-cumulative | |||||||
| RIG-4B | Block | Between the two tributaries | Non-cumulative | |||||||
| RIG-4C | Block | Between the two tributaries | Non-cumulative | |||||||
| RI-85 | In place | East of the crossroad | Non-cumulative | Sampled by S. Blais in 1994 | ||||||
| RI-86 | In place | West of the crossroad | Non-cumulative | Sampled by S. Blais in 1994 | ||||||
| Petrographic types . | Samples . | Outcropping . | Location . | Textural types . | Distinctive features . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ahititera pluton | ||||||||||
| Ultrabasic rocks | ||||||||||
| Olivine-clinopyroxenite | THG-5C | Clast | East of the pluton | Cumulate | ||||||
| THG-10B | In place | Ieifatautau | Cumulate | Lobate contact with THG-10C | ||||||
| Clinopyroxene-hornblendite | THG-2D | Block | Maroto | Cumulate | ||||||
| THG-14 | Block | Vaituoru | Cumulate | |||||||
| THG-7A | Clast | East of the pluton | Cumulate | |||||||
| Nepheline-free rocks = mildly Si-undersaturated rocks | ||||||||||
| Gabbro | THG-1B | Block | Maroto | Non-cumulative | ||||||
| THG-1 Da | Block | Maroto | Non-cumulative | Contains THG-1Db as a small dyke | ||||||
| THG-7B | Clast | East of the pluton | Non-cumulative | |||||||
| THG-10C | In place | Ieifatautau | Non-cumulative | Lobate contact with THG-10B | ||||||
| THG-10E | Block | Ieifatautau | Moderately cumulative | Layered | ||||||
| Monzonite | 37H | Block | Vaituoru | Non-cumulative | Sampled by R. Brousse and G. Guille in 1971 | |||||
| TP6 | Block | Papenoo | Non-cumulative | Sampled by J.-M. Bardintzeff in 1981 | ||||||
| Alkali syenite | THG-9B | In place | Near the Vaituoru dam | Non-cumulative | Hydrothermalized sample | |||||
| Nepheline-bearing rocks = strongly Si-undersaturated rocks | ||||||||||
| Theralite | THG-1A | Block | Maroto | Moderately cumulative | ||||||
| THG-2A | Block | Maroto | Moderately cumulative | |||||||
| THG-2B | Block | Maroto | Non-cumulative | |||||||
| THG-9E2 | Block | Vaituoru | Non-cumulative | Layered | ||||||
| THG-13A | Clast | East of the pluton | Non-cumulative | |||||||
| Essexite | THG-1E | Block | Maroto | Non-cumulative | ||||||
| THG-2C | Block | Maroto | Non-cumulative | |||||||
| THG-3A | Block | South of Maroto | Moderately cumulative | Contains THG-3B as a lobate vein | ||||||
| THG-9E1 | In place | Near the Vaituoru dam | Non-cumulative | |||||||
| THG-11A | Block | Ieifatautau | Moderately cumulative | |||||||
| Nepheline-monzosyenite | THG-4 | In place | South of Maroto | Non-cumulative | ||||||
| THG-6A | Clast | East of the pluton | Non-cumulative | |||||||
| Nepheline-syenite | THG-1Db | Block | Maroto | Non-cumulative | Small dyke in THG-1 Da | |||||
| THG-3B | Block | South of Maroto | Non-cumulative | Lobate vein in THG-3A | ||||||
| THG-19 | Clast | East of the pluton | Non-cumulative | |||||||
| Faaroa pluton | ||||||||||
| Nepheline-free rocks = mildly Si-undersaturated rocks | ||||||||||
| Gabbro | RIG-3B | In place | Apoomau: southern tributary | Non-cumulative | ||||||
| RI-28 | In place | Apoomau: southern tributary | Non-cumulative | Sampled by S. Blais in 1994 | ||||||
| Nepheline-bearing rocks = strongly Si-undersaturated rocks | ||||||||||
| Theralite | RIG-1B | In place | East of the crossroad | Non-cumulative | ||||||
| RIG-1E | In place | East of the crossroad | Non-cumulative | |||||||
| RIG-2A | In place | West of the crossroad | Non-cumulative | |||||||
| RIG-2C | In place | West of the crossroad | Non-cumulative | |||||||
| RIG-2D | In place | West of the crossroad | Non-cumulative | |||||||
| RIG-4A | In place | Apoomau: northern tributary | Non-cumulative | |||||||
| RIG-4B | Block | Between the two tributaries | Non-cumulative | |||||||
| RIG-4C | Block | Between the two tributaries | Non-cumulative | |||||||
| RI-85 | In place | East of the crossroad | Non-cumulative | Sampled by S. Blais in 1994 | ||||||
| RI-86 | In place | West of the crossroad | Non-cumulative | Sampled by S. Blais in 1994 | ||||||
The clasts have been collected within the debris avalanche deposits partially covering the Ahititera pluton.
Set of coarse-grained rocks sampled in Ahititera pluton (Tahiti Nui) and in Faaroa pluton (Raiatea)
| Petrographic types . | Samples . | Outcropping . | Location . | Textural types . | Distinctive features . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ahititera pluton | ||||||||||
| Ultrabasic rocks | ||||||||||
| Olivine-clinopyroxenite | THG-5C | Clast | East of the pluton | Cumulate | ||||||
| THG-10B | In place | Ieifatautau | Cumulate | Lobate contact with THG-10C | ||||||
| Clinopyroxene-hornblendite | THG-2D | Block | Maroto | Cumulate | ||||||
| THG-14 | Block | Vaituoru | Cumulate | |||||||
| THG-7A | Clast | East of the pluton | Cumulate | |||||||
| Nepheline-free rocks = mildly Si-undersaturated rocks | ||||||||||
| Gabbro | THG-1B | Block | Maroto | Non-cumulative | ||||||
| THG-1 Da | Block | Maroto | Non-cumulative | Contains THG-1Db as a small dyke | ||||||
| THG-7B | Clast | East of the pluton | Non-cumulative | |||||||
| THG-10C | In place | Ieifatautau | Non-cumulative | Lobate contact with THG-10B | ||||||
| THG-10E | Block | Ieifatautau | Moderately cumulative | Layered | ||||||
| Monzonite | 37H | Block | Vaituoru | Non-cumulative | Sampled by R. Brousse and G. Guille in 1971 | |||||
| TP6 | Block | Papenoo | Non-cumulative | Sampled by J.-M. Bardintzeff in 1981 | ||||||
| Alkali syenite | THG-9B | In place | Near the Vaituoru dam | Non-cumulative | Hydrothermalized sample | |||||
| Nepheline-bearing rocks = strongly Si-undersaturated rocks | ||||||||||
| Theralite | THG-1A | Block | Maroto | Moderately cumulative | ||||||
| THG-2A | Block | Maroto | Moderately cumulative | |||||||
| THG-2B | Block | Maroto | Non-cumulative | |||||||
| THG-9E2 | Block | Vaituoru | Non-cumulative | Layered | ||||||
| THG-13A | Clast | East of the pluton | Non-cumulative | |||||||
| Essexite | THG-1E | Block | Maroto | Non-cumulative | ||||||
| THG-2C | Block | Maroto | Non-cumulative | |||||||
| THG-3A | Block | South of Maroto | Moderately cumulative | Contains THG-3B as a lobate vein | ||||||
| THG-9E1 | In place | Near the Vaituoru dam | Non-cumulative | |||||||
| THG-11A | Block | Ieifatautau | Moderately cumulative | |||||||
| Nepheline-monzosyenite | THG-4 | In place | South of Maroto | Non-cumulative | ||||||
| THG-6A | Clast | East of the pluton | Non-cumulative | |||||||
| Nepheline-syenite | THG-1Db | Block | Maroto | Non-cumulative | Small dyke in THG-1 Da | |||||
| THG-3B | Block | South of Maroto | Non-cumulative | Lobate vein in THG-3A | ||||||
| THG-19 | Clast | East of the pluton | Non-cumulative | |||||||
| Faaroa pluton | ||||||||||
| Nepheline-free rocks = mildly Si-undersaturated rocks | ||||||||||
| Gabbro | RIG-3B | In place | Apoomau: southern tributary | Non-cumulative | ||||||
| RI-28 | In place | Apoomau: southern tributary | Non-cumulative | Sampled by S. Blais in 1994 | ||||||
| Nepheline-bearing rocks = strongly Si-undersaturated rocks | ||||||||||
| Theralite | RIG-1B | In place | East of the crossroad | Non-cumulative | ||||||
| RIG-1E | In place | East of the crossroad | Non-cumulative | |||||||
| RIG-2A | In place | West of the crossroad | Non-cumulative | |||||||
| RIG-2C | In place | West of the crossroad | Non-cumulative | |||||||
| RIG-2D | In place | West of the crossroad | Non-cumulative | |||||||
| RIG-4A | In place | Apoomau: northern tributary | Non-cumulative | |||||||
| RIG-4B | Block | Between the two tributaries | Non-cumulative | |||||||
| RIG-4C | Block | Between the two tributaries | Non-cumulative | |||||||
| RI-85 | In place | East of the crossroad | Non-cumulative | Sampled by S. Blais in 1994 | ||||||
| RI-86 | In place | West of the crossroad | Non-cumulative | Sampled by S. Blais in 1994 | ||||||
| Petrographic types . | Samples . | Outcropping . | Location . | Textural types . | Distinctive features . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ahititera pluton | ||||||||||
| Ultrabasic rocks | ||||||||||
| Olivine-clinopyroxenite | THG-5C | Clast | East of the pluton | Cumulate | ||||||
| THG-10B | In place | Ieifatautau | Cumulate | Lobate contact with THG-10C | ||||||
| Clinopyroxene-hornblendite | THG-2D | Block | Maroto | Cumulate | ||||||
| THG-14 | Block | Vaituoru | Cumulate | |||||||
| THG-7A | Clast | East of the pluton | Cumulate | |||||||
| Nepheline-free rocks = mildly Si-undersaturated rocks | ||||||||||
| Gabbro | THG-1B | Block | Maroto | Non-cumulative | ||||||
| THG-1 Da | Block | Maroto | Non-cumulative | Contains THG-1Db as a small dyke | ||||||
| THG-7B | Clast | East of the pluton | Non-cumulative | |||||||
| THG-10C | In place | Ieifatautau | Non-cumulative | Lobate contact with THG-10B | ||||||
| THG-10E | Block | Ieifatautau | Moderately cumulative | Layered | ||||||
| Monzonite | 37H | Block | Vaituoru | Non-cumulative | Sampled by R. Brousse and G. Guille in 1971 | |||||
| TP6 | Block | Papenoo | Non-cumulative | Sampled by J.-M. Bardintzeff in 1981 | ||||||
| Alkali syenite | THG-9B | In place | Near the Vaituoru dam | Non-cumulative | Hydrothermalized sample | |||||
| Nepheline-bearing rocks = strongly Si-undersaturated rocks | ||||||||||
| Theralite | THG-1A | Block | Maroto | Moderately cumulative | ||||||
| THG-2A | Block | Maroto | Moderately cumulative | |||||||
| THG-2B | Block | Maroto | Non-cumulative | |||||||
| THG-9E2 | Block | Vaituoru | Non-cumulative | Layered | ||||||
| THG-13A | Clast | East of the pluton | Non-cumulative | |||||||
| Essexite | THG-1E | Block | Maroto | Non-cumulative | ||||||
| THG-2C | Block | Maroto | Non-cumulative | |||||||
| THG-3A | Block | South of Maroto | Moderately cumulative | Contains THG-3B as a lobate vein | ||||||
| THG-9E1 | In place | Near the Vaituoru dam | Non-cumulative | |||||||
| THG-11A | Block | Ieifatautau | Moderately cumulative | |||||||
| Nepheline-monzosyenite | THG-4 | In place | South of Maroto | Non-cumulative | ||||||
| THG-6A | Clast | East of the pluton | Non-cumulative | |||||||
| Nepheline-syenite | THG-1Db | Block | Maroto | Non-cumulative | Small dyke in THG-1 Da | |||||
| THG-3B | Block | South of Maroto | Non-cumulative | Lobate vein in THG-3A | ||||||
| THG-19 | Clast | East of the pluton | Non-cumulative | |||||||
| Faaroa pluton | ||||||||||
| Nepheline-free rocks = mildly Si-undersaturated rocks | ||||||||||
| Gabbro | RIG-3B | In place | Apoomau: southern tributary | Non-cumulative | ||||||
| RI-28 | In place | Apoomau: southern tributary | Non-cumulative | Sampled by S. Blais in 1994 | ||||||
| Nepheline-bearing rocks = strongly Si-undersaturated rocks | ||||||||||
| Theralite | RIG-1B | In place | East of the crossroad | Non-cumulative | ||||||
| RIG-1E | In place | East of the crossroad | Non-cumulative | |||||||
| RIG-2A | In place | West of the crossroad | Non-cumulative | |||||||
| RIG-2C | In place | West of the crossroad | Non-cumulative | |||||||
| RIG-2D | In place | West of the crossroad | Non-cumulative | |||||||
| RIG-4A | In place | Apoomau: northern tributary | Non-cumulative | |||||||
| RIG-4B | Block | Between the two tributaries | Non-cumulative | |||||||
| RIG-4C | Block | Between the two tributaries | Non-cumulative | |||||||
| RI-85 | In place | East of the crossroad | Non-cumulative | Sampled by S. Blais in 1994 | ||||||
| RI-86 | In place | West of the crossroad | Non-cumulative | Sampled by S. Blais in 1994 | ||||||
The clasts have been collected within the debris avalanche deposits partially covering the Ahititera pluton.
Location of the studied samples. Four samples were collected during previous field trips [the Ahititera monzonite 37H: Bardintzeff et al. (1988) and the Faaroa gabbro RI-28, and theralites RI-85 and RI-86: Blais et al. (1997)]. Geological sketch maps of (a) the Ahititera plutonic body (Tahiti Nui) and its surroundings and (b) the Faaroa depression (Raiatea). Circled symbols are Raiatea samples.
Raiatea
The plutonic rocks of the Faaroa depression in Raiatea island, first observed by Deneufbourg (1965), were sampled by R. Brousse and E. Berger in the 1970s (Brousse & Berger, 1985) and by S. Blais and co-workers from 1994 to 1996 (Blais et al., 1997). The plutonic rocks are exposed in place within a c. 1 km2 east–west-trending area (Fig. 2b). The dense vegetation has only allowed sampling of two petrographic types (Table 1). Among the 14 coarse-grained mafic samples we collected in 1999, nine have been analysed for major and trace elements. They have been complemented with three rocks (RI-28, RI-85, and RI-86) sampled by S. Blais in 1994 (Blais et al., 1997), resulting in a total of 12 samples.
PETROLOGY AND MINERALOGY
The nomenclature of the coarse-grained rocks is based on Streckeisen's (1974) classification, modified by Le Bas & Streckeisen (1991). The modal (volumetric) proportions of minerals have been determined by point-counting on representative thin sections. The sample set can be divided into three main groups on the basis of petrographic criteria: an ultrabasic group and two others, which can be discriminated using the APF (alkali feldspar–plagioclase–feldspathoid) triangle of Fig. 3—a modal nepheline-free group and a modal nepheline-bearing group. Mineral compositions have been determined using a Cameca SX 50 automated electron microprobe (Microsonde Ouest, Brest, France). Analytical conditions were 15 kV, 15–20 nA, counting time 6 s, and correction by the ZAF method. Concentrations lower than 0·3 wt % are not considered representative. Modal proportions (transformed into weight percent) are shown in Table 2 and mineral compositions in Table 3 and Fig. 4.
Modal proportions (recalculated in weight percentages) for each petrographic type of the Tahiti-Nui samples
| . | Nepheline-free rocks . | . | . | Nepheline-bearing rocks . | . | . | . | . | . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Ol-Cpxite UB . | Gab . | Alk-Sye . | Cpx-Hblite UB . | The . | . | . | . | . | |||||||
. | Range (n = 2) . | Range (n = 6) . | THG-9B . | Range (n = 3) . | Range (n = 4) . | THG-2A . | THG-2A′ . | THG-1A . | THG-1A′ . | |||||||
| Olivine | 25·4–28·2 | 0–7·3 | Sparse | 0–3·5 | 12·5 | 13·7 | 27·6 | 23·5 | ||||||||
| Clinopyroxene | 57·8–58·4 | 33·9–41·8 | 6·0 | 14·7–32·4 | 11·3–24·3 | 42·7 | 14·0 | 23·3 | 14·6 | |||||||
| Fe–Ti oxides | 4·4 | 9·7–22·3 | 8·7 | 10·3–14·5 | 5·0–17·4 | 10·6 | 10·8 | |||||||||
| Plagioclase | 7·1–10·4 | 33·0–49·7 | 6·2–11·8 | 46·3–63·6 | 22·1 | 33·4 | ||||||||||
| K-Na feldspars | Sparse | 83·0 | 0–0·8 | 0–1·7 | 1·6 | |||||||||||
| Nepheline | 0·8–5·6 | 4·3–8·6 | 4·1 | |||||||||||||
| Hornblende | 0–1·5 | 0–8·3 | 36·5–59·9 | 0·5–4·5 | 0·5 | |||||||||||
| Biotite | 0·5–1·8 | 0–3·0 | 1·1 | 0·9 | 1·1–5·2 | 3·8 | 3·8 | |||||||||
| Apatite | 0–2·1 | 1·2 | 3·8–5·8 | 1·1–4·4 | 2·0 | 1·0 | ||||||||||
| Titanite | Sparse | |||||||||||||||
| Liquid | 71·8 | 62·3 | ||||||||||||||
| ΣR2 | 0·4 | 0·1 | ||||||||||||||
| . | Nepheline-free rocks . | . | . | Nepheline-bearing rocks . | . | . | . | . | . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Ol-Cpxite UB . | Gab . | Alk-Sye . | Cpx-Hblite UB . | The . | . | . | . | . | |||||||
. | Range (n = 2) . | Range (n = 6) . | THG-9B . | Range (n = 3) . | Range (n = 4) . | THG-2A . | THG-2A′ . | THG-1A . | THG-1A′ . | |||||||
| Olivine | 25·4–28·2 | 0–7·3 | Sparse | 0–3·5 | 12·5 | 13·7 | 27·6 | 23·5 | ||||||||
| Clinopyroxene | 57·8–58·4 | 33·9–41·8 | 6·0 | 14·7–32·4 | 11·3–24·3 | 42·7 | 14·0 | 23·3 | 14·6 | |||||||
| Fe–Ti oxides | 4·4 | 9·7–22·3 | 8·7 | 10·3–14·5 | 5·0–17·4 | 10·6 | 10·8 | |||||||||
| Plagioclase | 7·1–10·4 | 33·0–49·7 | 6·2–11·8 | 46·3–63·6 | 22·1 | 33·4 | ||||||||||
| K-Na feldspars | Sparse | 83·0 | 0–0·8 | 0–1·7 | 1·6 | |||||||||||
| Nepheline | 0·8–5·6 | 4·3–8·6 | 4·1 | |||||||||||||
| Hornblende | 0–1·5 | 0–8·3 | 36·5–59·9 | 0·5–4·5 | 0·5 | |||||||||||
| Biotite | 0·5–1·8 | 0–3·0 | 1·1 | 0·9 | 1·1–5·2 | 3·8 | 3·8 | |||||||||
| Apatite | 0–2·1 | 1·2 | 3·8–5·8 | 1·1–4·4 | 2·0 | 1·0 | ||||||||||
| Titanite | Sparse | |||||||||||||||
| Liquid | 71·8 | 62·3 | ||||||||||||||
| ΣR2 | 0·4 | 0·1 | ||||||||||||||
| . | Nepheline-bearing rocks . | . | . | . | . | . | . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Ess . | . | . | . | . | Ne-Msye . | Ne-Sye . | |||||||
. | Range (n = 3) . | THG-3A . | THG-3A′ . | THG-11A . | THG-11A′ . | Range (n = 2) . | Range (n = 3) . | |||||||
| Olivine | Sparse | 1·1 | 1·5 | 1·1 | 1·5 | |||||||||
| Clinopyroxene | 3·4–15·2 | 9·7 | 24·6 | 29·0 | 19·4 | 4·6–7·0 | 3·6–4·7 | |||||||
| Fe–Ti oxides | 5·0–11·0 | 6·3 | 4·7 | 9·4 | 4·1 | 6·7–8·5 | 3·5–8·5 | |||||||
| Plagioclase | 16·2–26·8 | 19·6 | 16·1 | 14·6–22·0 | 0·9–3·8 | |||||||||
| K-Na feldspars | 12·8–18·8 | 11·1 | 12·7 | 32·1–40·1 | 54·4–63·1 | |||||||||
| Nepheline | 11·1–14·5 | 6·8 | 9·3 | 10·9–12·9 | 17·2–17·5 | |||||||||
| Hornblende | 21·7–35·7 | 41·1 | 19·3 | 14·0–19·6 | 2·4–9·4 | |||||||||
| Biotite | ||||||||||||||
| Apatite | 3·1–4·2 | 3·1 | 2·1 | 2·2–2·3 | 2·4–3·4 | |||||||||
| Titanite | 2·3–3·5 | 1·1 | 1·1 | 1·2 | 1·2–3·9 | |||||||||
| Liquid | 69·4 | 74·2 | ||||||||||||
| ΣR2 | 0·5 | 0·3 | ||||||||||||
| . | Nepheline-bearing rocks . | . | . | . | . | . | . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Ess . | . | . | . | . | Ne-Msye . | Ne-Sye . | |||||||
. | Range (n = 3) . | THG-3A . | THG-3A′ . | THG-11A . | THG-11A′ . | Range (n = 2) . | Range (n = 3) . | |||||||
| Olivine | Sparse | 1·1 | 1·5 | 1·1 | 1·5 | |||||||||
| Clinopyroxene | 3·4–15·2 | 9·7 | 24·6 | 29·0 | 19·4 | 4·6–7·0 | 3·6–4·7 | |||||||
| Fe–Ti oxides | 5·0–11·0 | 6·3 | 4·7 | 9·4 | 4·1 | 6·7–8·5 | 3·5–8·5 | |||||||
| Plagioclase | 16·2–26·8 | 19·6 | 16·1 | 14·6–22·0 | 0·9–3·8 | |||||||||
| K-Na feldspars | 12·8–18·8 | 11·1 | 12·7 | 32·1–40·1 | 54·4–63·1 | |||||||||
| Nepheline | 11·1–14·5 | 6·8 | 9·3 | 10·9–12·9 | 17·2–17·5 | |||||||||
| Hornblende | 21·7–35·7 | 41·1 | 19·3 | 14·0–19·6 | 2·4–9·4 | |||||||||
| Biotite | ||||||||||||||
| Apatite | 3·1–4·2 | 3·1 | 2·1 | 2·2–2·3 | 2·4–3·4 | |||||||||
| Titanite | 2·3–3·5 | 1·1 | 1·1 | 1·2 | 1·2–3·9 | |||||||||
| Liquid | 69·4 | 74·2 | ||||||||||||
| ΣR2 | 0·5 | 0·3 | ||||||||||||
Ol-Cpxite, olivine-clinopyroxenite; Gab, gabbro; Alk-Sye, alkali-syenite; Cpx-Hblite, clinopyroxene-hornblendite; The, theralite; Ess, essexite; Ne-Msye, nepheline-monzosyenite; Ne-Sye, nepheline-syenite. n, number of samples. UB, ultrabasic rocks. The ‘prime’ symbol attached to the name of moderately cumulative theralites and essexites indicates theoretical modal compositions, including a liquid phase together with cumulus minerals, reconstructed using the MONA program (see text for explanation). ΣR2, sum of the squared residuals.
Modal proportions (recalculated in weight percentages) for each petrographic type of the Tahiti-Nui samples
| . | Nepheline-free rocks . | . | . | Nepheline-bearing rocks . | . | . | . | . | . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Ol-Cpxite UB . | Gab . | Alk-Sye . | Cpx-Hblite UB . | The . | . | . | . | . | |||||||
. | Range (n = 2) . | Range (n = 6) . | THG-9B . | Range (n = 3) . | Range (n = 4) . | THG-2A . | THG-2A′ . | THG-1A . | THG-1A′ . | |||||||
| Olivine | 25·4–28·2 | 0–7·3 | Sparse | 0–3·5 | 12·5 | 13·7 | 27·6 | 23·5 | ||||||||
| Clinopyroxene | 57·8–58·4 | 33·9–41·8 | 6·0 | 14·7–32·4 | 11·3–24·3 | 42·7 | 14·0 | 23·3 | 14·6 | |||||||
| Fe–Ti oxides | 4·4 | 9·7–22·3 | 8·7 | 10·3–14·5 | 5·0–17·4 | 10·6 | 10·8 | |||||||||
| Plagioclase | 7·1–10·4 | 33·0–49·7 | 6·2–11·8 | 46·3–63·6 | 22·1 | 33·4 | ||||||||||
| K-Na feldspars | Sparse | 83·0 | 0–0·8 | 0–1·7 | 1·6 | |||||||||||
| Nepheline | 0·8–5·6 | 4·3–8·6 | 4·1 | |||||||||||||
| Hornblende | 0–1·5 | 0–8·3 | 36·5–59·9 | 0·5–4·5 | 0·5 | |||||||||||
| Biotite | 0·5–1·8 | 0–3·0 | 1·1 | 0·9 | 1·1–5·2 | 3·8 | 3·8 | |||||||||
| Apatite | 0–2·1 | 1·2 | 3·8–5·8 | 1·1–4·4 | 2·0 | 1·0 | ||||||||||
| Titanite | Sparse | |||||||||||||||
| Liquid | 71·8 | 62·3 | ||||||||||||||
| ΣR2 | 0·4 | 0·1 | ||||||||||||||
| . | Nepheline-free rocks . | . | . | Nepheline-bearing rocks . | . | . | . | . | . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Ol-Cpxite UB . | Gab . | Alk-Sye . | Cpx-Hblite UB . | The . | . | . | . | . | |||||||
. | Range (n = 2) . | Range (n = 6) . | THG-9B . | Range (n = 3) . | Range (n = 4) . | THG-2A . | THG-2A′ . | THG-1A . | THG-1A′ . | |||||||
| Olivine | 25·4–28·2 | 0–7·3 | Sparse | 0–3·5 | 12·5 | 13·7 | 27·6 | 23·5 | ||||||||
| Clinopyroxene | 57·8–58·4 | 33·9–41·8 | 6·0 | 14·7–32·4 | 11·3–24·3 | 42·7 | 14·0 | 23·3 | 14·6 | |||||||
| Fe–Ti oxides | 4·4 | 9·7–22·3 | 8·7 | 10·3–14·5 | 5·0–17·4 | 10·6 | 10·8 | |||||||||
| Plagioclase | 7·1–10·4 | 33·0–49·7 | 6·2–11·8 | 46·3–63·6 | 22·1 | 33·4 | ||||||||||
| K-Na feldspars | Sparse | 83·0 | 0–0·8 | 0–1·7 | 1·6 | |||||||||||
| Nepheline | 0·8–5·6 | 4·3–8·6 | 4·1 | |||||||||||||
| Hornblende | 0–1·5 | 0–8·3 | 36·5–59·9 | 0·5–4·5 | 0·5 | |||||||||||
| Biotite | 0·5–1·8 | 0–3·0 | 1·1 | 0·9 | 1·1–5·2 | 3·8 | 3·8 | |||||||||
| Apatite | 0–2·1 | 1·2 | 3·8–5·8 | 1·1–4·4 | 2·0 | 1·0 | ||||||||||
| Titanite | Sparse | |||||||||||||||
| Liquid | 71·8 | 62·3 | ||||||||||||||
| ΣR2 | 0·4 | 0·1 | ||||||||||||||
| . | Nepheline-bearing rocks . | . | . | . | . | . | . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Ess . | . | . | . | . | Ne-Msye . | Ne-Sye . | |||||||
. | Range (n = 3) . | THG-3A . | THG-3A′ . | THG-11A . | THG-11A′ . | Range (n = 2) . | Range (n = 3) . | |||||||
| Olivine | Sparse | 1·1 | 1·5 | 1·1 | 1·5 | |||||||||
| Clinopyroxene | 3·4–15·2 | 9·7 | 24·6 | 29·0 | 19·4 | 4·6–7·0 | 3·6–4·7 | |||||||
| Fe–Ti oxides | 5·0–11·0 | 6·3 | 4·7 | 9·4 | 4·1 | 6·7–8·5 | 3·5–8·5 | |||||||
| Plagioclase | 16·2–26·8 | 19·6 | 16·1 | 14·6–22·0 | 0·9–3·8 | |||||||||
| K-Na feldspars | 12·8–18·8 | 11·1 | 12·7 | 32·1–40·1 | 54·4–63·1 | |||||||||
| Nepheline | 11·1–14·5 | 6·8 | 9·3 | 10·9–12·9 | 17·2–17·5 | |||||||||
| Hornblende | 21·7–35·7 | 41·1 | 19·3 | 14·0–19·6 | 2·4–9·4 | |||||||||
| Biotite | ||||||||||||||
| Apatite | 3·1–4·2 | 3·1 | 2·1 | 2·2–2·3 | 2·4–3·4 | |||||||||
| Titanite | 2·3–3·5 | 1·1 | 1·1 | 1·2 | 1·2–3·9 | |||||||||
| Liquid | 69·4 | 74·2 | ||||||||||||
| ΣR2 | 0·5 | 0·3 | ||||||||||||
| . | Nepheline-bearing rocks . | . | . | . | . | . | . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Ess . | . | . | . | . | Ne-Msye . | Ne-Sye . | |||||||
. | Range (n = 3) . | THG-3A . | THG-3A′ . | THG-11A . | THG-11A′ . | Range (n = 2) . | Range (n = 3) . | |||||||
| Olivine | Sparse | 1·1 | 1·5 | 1·1 | 1·5 | |||||||||
| Clinopyroxene | 3·4–15·2 | 9·7 | 24·6 | 29·0 | 19·4 | 4·6–7·0 | 3·6–4·7 | |||||||
| Fe–Ti oxides | 5·0–11·0 | 6·3 | 4·7 | 9·4 | 4·1 | 6·7–8·5 | 3·5–8·5 | |||||||
| Plagioclase | 16·2–26·8 | 19·6 | 16·1 | 14·6–22·0 | 0·9–3·8 | |||||||||
| K-Na feldspars | 12·8–18·8 | 11·1 | 12·7 | 32·1–40·1 | 54·4–63·1 | |||||||||
| Nepheline | 11·1–14·5 | 6·8 | 9·3 | 10·9–12·9 | 17·2–17·5 | |||||||||
| Hornblende | 21·7–35·7 | 41·1 | 19·3 | 14·0–19·6 | 2·4–9·4 | |||||||||
| Biotite | ||||||||||||||
| Apatite | 3·1–4·2 | 3·1 | 2·1 | 2·2–2·3 | 2·4–3·4 | |||||||||
| Titanite | 2·3–3·5 | 1·1 | 1·1 | 1·2 | 1·2–3·9 | |||||||||
| Liquid | 69·4 | 74·2 | ||||||||||||
| ΣR2 | 0·5 | 0·3 | ||||||||||||
Ol-Cpxite, olivine-clinopyroxenite; Gab, gabbro; Alk-Sye, alkali-syenite; Cpx-Hblite, clinopyroxene-hornblendite; The, theralite; Ess, essexite; Ne-Msye, nepheline-monzosyenite; Ne-Sye, nepheline-syenite. n, number of samples. UB, ultrabasic rocks. The ‘prime’ symbol attached to the name of moderately cumulative theralites and essexites indicates theoretical modal compositions, including a liquid phase together with cumulus minerals, reconstructed using the MONA program (see text for explanation). ΣR2, sum of the squared residuals.
Selected clinopyroxene, amphibole and plagioclase analyses from Ahititera pluton (Tahiti-Nui)
| Mineral: . | Cpx . | Cpx . | Cpx . | Cpx . | Hbl . | Hbl . | Hbl . | Hbl . | Hbl . | Hbl . | Plg . | Plg . | Plg . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Di . | Di . | Heden . | Heden . | Kaer . | Fe-Parg . | Kaer . | Kaer . | Mg-Hast . | Fe-Kaer . | Labrad . | Andes . | Bytow . |
| . | core . | core . | rim . | rim . | core . | rim . | replacing . | core . | rim . | replacing . | core . | rim . | core . |
| Rock: . | The . | Ess . | Ess . | Ess . | Ess . | Ess . | Ess . | Ne-Msye . | Ne-Msye . | Ne-Msye . | The . | The . | Ess . |
| Sample: . | THG-13A . | THG-9E1 . | THG-9E1 . | THG-9E1 . | THG-3A . | THG-3A . | THG-3A . | THG-19 . | THG-19 . | THG-19 . | THG-13A . | THG-13A . | THG-11A . |
| SiO2 | 51·48 | 45·45 | 49·23 | 48·43 | 39·30 | 38·84 | 39·19 | 38·94 | 39·72 | 38·73 | 53·15 | 57·09 | 50·57 |
| TiO2 | 0·49 | 3·52 | 1·20 | 0·69 | 7·06 | 3·08 | 4·63 | 6·28 | 1·29 | 4·66 | 0·15 | 0·15 | 0·03 |
| Al2O3 | 2·95 | 7·92 | 3·04 | 2·43 | 12·72 | 11·73 | 11·82 | 12·94 | 10·10 | 12·42 | 29·20 | 26·67 | 30·69 |
| FeO | 10·03 | 7·58 | 15·48 | 18·93 | 10·46 | 19·15 | 15·88 | 14·55 | 21·68 | 15·62 | 0·25 | 0·13 | 0·49 |
| MnO | 0·33 | 0·19 | 0·60 | 1·18 | 0·24 | 0·54 | 0·48 | 0·39 | 2·13 | 0·91 | 0·01 | 0·00 | 0·00 |
| MgO | 11·80 | 11·68 | 7·95 | 5·48 | 11·68 | 7·93 | 9·76 | 10·34 | 7·04 | 8·70 | 0·00 | 0·02 | 0·02 |
| CaO | 21·94 | 23·52 | 21·52 | 21·00 | 11·78 | 11·10 | 11·66 | 12·10 | 10·59 | 11·43 | 12·35 | 9·56 | 4·66 |
| Na2O | 1·17 | 0·56 | 1·51 | 1·63 | 2·72 | 2·68 | 2·68 | 2·63 | 2·89 | 2·67 | 4·49 | 6·14 | 3·37 |
| K2O | 0·02 | 0·00 | 0·00 | 0·00 | 1·33 | 1·94 | 1·91 | 1·57 | 1·77 | 1·90 | 0·03 | 0·18 | 0·13 |
| P2O5 | 0·00 | 0·03 | 0·02 | 0·10 | 0·02 | 0·03 | 0·00 | 0·00 | 0·07 | 0·00 | 0·05 | 0·00 | 0·04 |
| Cr2O3 | 0·00 | 0·00 | 0·00 | 0·00 | 0·00 | 0·03 | 0·00 | 0·00 | 0·03 | 0·00 | 0·10 | 0·00 | 0·03 |
| NiO | 0·00 | 0·00 | 0·00 | 0·17 | 0·04 | 0·01 | 0·06 | 0·00 | 0·11 | 0·00 | 0·06 | 0·03 | 0·00 |
| Total | 100·19 | 100·45 | 100·55 | 100·03 | 97·35 | 97·06 | 98·07 | 99·73 | 97·41 | 97·02 | 99·82 | 99·96 | 100·02 |
| Fe3+ | 0·091 | 0·118 | 0·155 | 0·190 | 0·000 | 0·083 | 0·000 | 0·000 | 0·612 | 0·000 | 0·009 | 0·005 | 0·019 |
| Fe2+ | 0·22 | 0·12 | 0·34 | 0·43 | 1·31 | 2·42 | 2·03 | 1·81 | 2·23 | 2·01 | |||
| Mg/(Mg + Fe2+) | 0·74 | 0·84 | 0·56 | 0·41 | 0·67 | 0·43 | 0·52 | 0·56 | 0·42 | 0·50 | |||
| Wo | 47·24 | 51·31 | 47·67 | 47·37 | |||||||||
| En | 35·34 | 35·46 | 24·50 | 17·19 | |||||||||
| Fs | 17·42 | 13·24 | 27·83 | 35·43 | |||||||||
| An | 60·22 | 45·78 | 70·11 |
| Mineral: . | Cpx . | Cpx . | Cpx . | Cpx . | Hbl . | Hbl . | Hbl . | Hbl . | Hbl . | Hbl . | Plg . | Plg . | Plg . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Di . | Di . | Heden . | Heden . | Kaer . | Fe-Parg . | Kaer . | Kaer . | Mg-Hast . | Fe-Kaer . | Labrad . | Andes . | Bytow . |
| . | core . | core . | rim . | rim . | core . | rim . | replacing . | core . | rim . | replacing . | core . | rim . | core . |
| Rock: . | The . | Ess . | Ess . | Ess . | Ess . | Ess . | Ess . | Ne-Msye . | Ne-Msye . | Ne-Msye . | The . | The . | Ess . |
| Sample: . | THG-13A . | THG-9E1 . | THG-9E1 . | THG-9E1 . | THG-3A . | THG-3A . | THG-3A . | THG-19 . | THG-19 . | THG-19 . | THG-13A . | THG-13A . | THG-11A . |
| SiO2 | 51·48 | 45·45 | 49·23 | 48·43 | 39·30 | 38·84 | 39·19 | 38·94 | 39·72 | 38·73 | 53·15 | 57·09 | 50·57 |
| TiO2 | 0·49 | 3·52 | 1·20 | 0·69 | 7·06 | 3·08 | 4·63 | 6·28 | 1·29 | 4·66 | 0·15 | 0·15 | 0·03 |
| Al2O3 | 2·95 | 7·92 | 3·04 | 2·43 | 12·72 | 11·73 | 11·82 | 12·94 | 10·10 | 12·42 | 29·20 | 26·67 | 30·69 |
| FeO | 10·03 | 7·58 | 15·48 | 18·93 | 10·46 | 19·15 | 15·88 | 14·55 | 21·68 | 15·62 | 0·25 | 0·13 | 0·49 |
| MnO | 0·33 | 0·19 | 0·60 | 1·18 | 0·24 | 0·54 | 0·48 | 0·39 | 2·13 | 0·91 | 0·01 | 0·00 | 0·00 |
| MgO | 11·80 | 11·68 | 7·95 | 5·48 | 11·68 | 7·93 | 9·76 | 10·34 | 7·04 | 8·70 | 0·00 | 0·02 | 0·02 |
| CaO | 21·94 | 23·52 | 21·52 | 21·00 | 11·78 | 11·10 | 11·66 | 12·10 | 10·59 | 11·43 | 12·35 | 9·56 | 4·66 |
| Na2O | 1·17 | 0·56 | 1·51 | 1·63 | 2·72 | 2·68 | 2·68 | 2·63 | 2·89 | 2·67 | 4·49 | 6·14 | 3·37 |
| K2O | 0·02 | 0·00 | 0·00 | 0·00 | 1·33 | 1·94 | 1·91 | 1·57 | 1·77 | 1·90 | 0·03 | 0·18 | 0·13 |
| P2O5 | 0·00 | 0·03 | 0·02 | 0·10 | 0·02 | 0·03 | 0·00 | 0·00 | 0·07 | 0·00 | 0·05 | 0·00 | 0·04 |
| Cr2O3 | 0·00 | 0·00 | 0·00 | 0·00 | 0·00 | 0·03 | 0·00 | 0·00 | 0·03 | 0·00 | 0·10 | 0·00 | 0·03 |
| NiO | 0·00 | 0·00 | 0·00 | 0·17 | 0·04 | 0·01 | 0·06 | 0·00 | 0·11 | 0·00 | 0·06 | 0·03 | 0·00 |
| Total | 100·19 | 100·45 | 100·55 | 100·03 | 97·35 | 97·06 | 98·07 | 99·73 | 97·41 | 97·02 | 99·82 | 99·96 | 100·02 |
| Fe3+ | 0·091 | 0·118 | 0·155 | 0·190 | 0·000 | 0·083 | 0·000 | 0·000 | 0·612 | 0·000 | 0·009 | 0·005 | 0·019 |
| Fe2+ | 0·22 | 0·12 | 0·34 | 0·43 | 1·31 | 2·42 | 2·03 | 1·81 | 2·23 | 2·01 | |||
| Mg/(Mg + Fe2+) | 0·74 | 0·84 | 0·56 | 0·41 | 0·67 | 0·43 | 0·52 | 0·56 | 0·42 | 0·50 | |||
| Wo | 47·24 | 51·31 | 47·67 | 47·37 | |||||||||
| En | 35·34 | 35·46 | 24·50 | 17·19 | |||||||||
| Fs | 17·42 | 13·24 | 27·83 | 35·43 | |||||||||
| An | 60·22 | 45·78 | 70·11 |
Fe3+ and Fe2+ are expressed as cations per formula unit. All the iron of plagioclase is reported as Fe3+. For amphibole, the term ‘replacing’ denotes partial replacement of clinopyroxene rim by late hornblende. Abbreviations as in Table 1 and as follows: Cpx, clinopyroxene; Hbl, hornblende; Plg, plagioclase; Di, diopside; Heden, hedenbergite; Kaer, kaersutite; Fe-Parg, ferroan pargasite; Mg-Hast, magnesian hastingsite; Fe-Kaer, ferro-kaersutite; Labrad, labradorite; Andes, andesine; Bytow, bytownite.
Selected clinopyroxene, amphibole and plagioclase analyses from Ahititera pluton (Tahiti-Nui)
| Mineral: . | Cpx . | Cpx . | Cpx . | Cpx . | Hbl . | Hbl . | Hbl . | Hbl . | Hbl . | Hbl . | Plg . | Plg . | Plg . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Di . | Di . | Heden . | Heden . | Kaer . | Fe-Parg . | Kaer . | Kaer . | Mg-Hast . | Fe-Kaer . | Labrad . | Andes . | Bytow . |
| . | core . | core . | rim . | rim . | core . | rim . | replacing . | core . | rim . | replacing . | core . | rim . | core . |
| Rock: . | The . | Ess . | Ess . | Ess . | Ess . | Ess . | Ess . | Ne-Msye . | Ne-Msye . | Ne-Msye . | The . | The . | Ess . |
| Sample: . | THG-13A . | THG-9E1 . | THG-9E1 . | THG-9E1 . | THG-3A . | THG-3A . | THG-3A . | THG-19 . | THG-19 . | THG-19 . | THG-13A . | THG-13A . | THG-11A . |
| SiO2 | 51·48 | 45·45 | 49·23 | 48·43 | 39·30 | 38·84 | 39·19 | 38·94 | 39·72 | 38·73 | 53·15 | 57·09 | 50·57 |
| TiO2 | 0·49 | 3·52 | 1·20 | 0·69 | 7·06 | 3·08 | 4·63 | 6·28 | 1·29 | 4·66 | 0·15 | 0·15 | 0·03 |
| Al2O3 | 2·95 | 7·92 | 3·04 | 2·43 | 12·72 | 11·73 | 11·82 | 12·94 | 10·10 | 12·42 | 29·20 | 26·67 | 30·69 |
| FeO | 10·03 | 7·58 | 15·48 | 18·93 | 10·46 | 19·15 | 15·88 | 14·55 | 21·68 | 15·62 | 0·25 | 0·13 | 0·49 |
| MnO | 0·33 | 0·19 | 0·60 | 1·18 | 0·24 | 0·54 | 0·48 | 0·39 | 2·13 | 0·91 | 0·01 | 0·00 | 0·00 |
| MgO | 11·80 | 11·68 | 7·95 | 5·48 | 11·68 | 7·93 | 9·76 | 10·34 | 7·04 | 8·70 | 0·00 | 0·02 | 0·02 |
| CaO | 21·94 | 23·52 | 21·52 | 21·00 | 11·78 | 11·10 | 11·66 | 12·10 | 10·59 | 11·43 | 12·35 | 9·56 | 4·66 |
| Na2O | 1·17 | 0·56 | 1·51 | 1·63 | 2·72 | 2·68 | 2·68 | 2·63 | 2·89 | 2·67 | 4·49 | 6·14 | 3·37 |
| K2O | 0·02 | 0·00 | 0·00 | 0·00 | 1·33 | 1·94 | 1·91 | 1·57 | 1·77 | 1·90 | 0·03 | 0·18 | 0·13 |
| P2O5 | 0·00 | 0·03 | 0·02 | 0·10 | 0·02 | 0·03 | 0·00 | 0·00 | 0·07 | 0·00 | 0·05 | 0·00 | 0·04 |
| Cr2O3 | 0·00 | 0·00 | 0·00 | 0·00 | 0·00 | 0·03 | 0·00 | 0·00 | 0·03 | 0·00 | 0·10 | 0·00 | 0·03 |
| NiO | 0·00 | 0·00 | 0·00 | 0·17 | 0·04 | 0·01 | 0·06 | 0·00 | 0·11 | 0·00 | 0·06 | 0·03 | 0·00 |
| Total | 100·19 | 100·45 | 100·55 | 100·03 | 97·35 | 97·06 | 98·07 | 99·73 | 97·41 | 97·02 | 99·82 | 99·96 | 100·02 |
| Fe3+ | 0·091 | 0·118 | 0·155 | 0·190 | 0·000 | 0·083 | 0·000 | 0·000 | 0·612 | 0·000 | 0·009 | 0·005 | 0·019 |
| Fe2+ | 0·22 | 0·12 | 0·34 | 0·43 | 1·31 | 2·42 | 2·03 | 1·81 | 2·23 | 2·01 | |||
| Mg/(Mg + Fe2+) | 0·74 | 0·84 | 0·56 | 0·41 | 0·67 | 0·43 | 0·52 | 0·56 | 0·42 | 0·50 | |||
| Wo | 47·24 | 51·31 | 47·67 | 47·37 | |||||||||
| En | 35·34 | 35·46 | 24·50 | 17·19 | |||||||||
| Fs | 17·42 | 13·24 | 27·83 | 35·43 | |||||||||
| An | 60·22 | 45·78 | 70·11 |
| Mineral: . | Cpx . | Cpx . | Cpx . | Cpx . | Hbl . | Hbl . | Hbl . | Hbl . | Hbl . | Hbl . | Plg . | Plg . | Plg . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Di . | Di . | Heden . | Heden . | Kaer . | Fe-Parg . | Kaer . | Kaer . | Mg-Hast . | Fe-Kaer . | Labrad . | Andes . | Bytow . |
| . | core . | core . | rim . | rim . | core . | rim . | replacing . | core . | rim . | replacing . | core . | rim . | core . |
| Rock: . | The . | Ess . | Ess . | Ess . | Ess . | Ess . | Ess . | Ne-Msye . | Ne-Msye . | Ne-Msye . | The . | The . | Ess . |
| Sample: . | THG-13A . | THG-9E1 . | THG-9E1 . | THG-9E1 . | THG-3A . | THG-3A . | THG-3A . | THG-19 . | THG-19 . | THG-19 . | THG-13A . | THG-13A . | THG-11A . |
| SiO2 | 51·48 | 45·45 | 49·23 | 48·43 | 39·30 | 38·84 | 39·19 | 38·94 | 39·72 | 38·73 | 53·15 | 57·09 | 50·57 |
| TiO2 | 0·49 | 3·52 | 1·20 | 0·69 | 7·06 | 3·08 | 4·63 | 6·28 | 1·29 | 4·66 | 0·15 | 0·15 | 0·03 |
| Al2O3 | 2·95 | 7·92 | 3·04 | 2·43 | 12·72 | 11·73 | 11·82 | 12·94 | 10·10 | 12·42 | 29·20 | 26·67 | 30·69 |
| FeO | 10·03 | 7·58 | 15·48 | 18·93 | 10·46 | 19·15 | 15·88 | 14·55 | 21·68 | 15·62 | 0·25 | 0·13 | 0·49 |
| MnO | 0·33 | 0·19 | 0·60 | 1·18 | 0·24 | 0·54 | 0·48 | 0·39 | 2·13 | 0·91 | 0·01 | 0·00 | 0·00 |
| MgO | 11·80 | 11·68 | 7·95 | 5·48 | 11·68 | 7·93 | 9·76 | 10·34 | 7·04 | 8·70 | 0·00 | 0·02 | 0·02 |
| CaO | 21·94 | 23·52 | 21·52 | 21·00 | 11·78 | 11·10 | 11·66 | 12·10 | 10·59 | 11·43 | 12·35 | 9·56 | 4·66 |
| Na2O | 1·17 | 0·56 | 1·51 | 1·63 | 2·72 | 2·68 | 2·68 | 2·63 | 2·89 | 2·67 | 4·49 | 6·14 | 3·37 |
| K2O | 0·02 | 0·00 | 0·00 | 0·00 | 1·33 | 1·94 | 1·91 | 1·57 | 1·77 | 1·90 | 0·03 | 0·18 | 0·13 |
| P2O5 | 0·00 | 0·03 | 0·02 | 0·10 | 0·02 | 0·03 | 0·00 | 0·00 | 0·07 | 0·00 | 0·05 | 0·00 | 0·04 |
| Cr2O3 | 0·00 | 0·00 | 0·00 | 0·00 | 0·00 | 0·03 | 0·00 | 0·00 | 0·03 | 0·00 | 0·10 | 0·00 | 0·03 |
| NiO | 0·00 | 0·00 | 0·00 | 0·17 | 0·04 | 0·01 | 0·06 | 0·00 | 0·11 | 0·00 | 0·06 | 0·03 | 0·00 |
| Total | 100·19 | 100·45 | 100·55 | 100·03 | 97·35 | 97·06 | 98·07 | 99·73 | 97·41 | 97·02 | 99·82 | 99·96 | 100·02 |
| Fe3+ | 0·091 | 0·118 | 0·155 | 0·190 | 0·000 | 0·083 | 0·000 | 0·000 | 0·612 | 0·000 | 0·009 | 0·005 | 0·019 |
| Fe2+ | 0·22 | 0·12 | 0·34 | 0·43 | 1·31 | 2·42 | 2·03 | 1·81 | 2·23 | 2·01 | |||
| Mg/(Mg + Fe2+) | 0·74 | 0·84 | 0·56 | 0·41 | 0·67 | 0·43 | 0·52 | 0·56 | 0·42 | 0·50 | |||
| Wo | 47·24 | 51·31 | 47·67 | 47·37 | |||||||||
| En | 35·34 | 35·46 | 24·50 | 17·19 | |||||||||
| Fs | 17·42 | 13·24 | 27·83 | 35·43 | |||||||||
| An | 60·22 | 45·78 | 70·11 |
Fe3+ and Fe2+ are expressed as cations per formula unit. All the iron of plagioclase is reported as Fe3+. For amphibole, the term ‘replacing’ denotes partial replacement of clinopyroxene rim by late hornblende. Abbreviations as in Table 1 and as follows: Cpx, clinopyroxene; Hbl, hornblende; Plg, plagioclase; Di, diopside; Heden, hedenbergite; Kaer, kaersutite; Fe-Parg, ferroan pargasite; Mg-Hast, magnesian hastingsite; Fe-Kaer, ferro-kaersutite; Labrad, labradorite; Andes, andesine; Bytow, bytownite.
Classification of the coarse-grained rocks of Ahititera and Faaroa according to their modal mineral contents (volume proportions). Triangle APF for plutonic rocks bearing felsic minerals (Streckeisen, 1974; Le Bas & Streckeisen, 1991): Q, quartz; A, alkali feldspar; P, plagioclase; F, feldspathoid. Triangle Plg–Ol–Cpx for gabbroic rocks (Streckeisen, 1976; Le Bas & Streckeisen, 1991): Plg, plagioclase; Ol, olivine; Cpx, pyroxene. Triangle Ol–Hbl–Cpx for ultrabasic rocks (Streckeisen, 1973; Le Bas & Streckeisen, 1991): M, mafic (non-QAPF) minerals; Hbl, hornblende. Circled symbols are Raiatea samples.
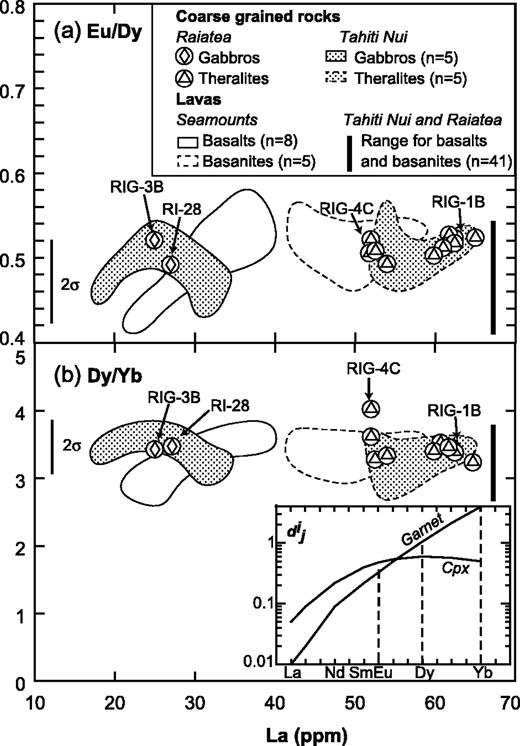
Summary of petrographic data for Tahiti Nui and Raiatea samples: mineral occurrences (continuous lines, main minerals, generally euhedral; dashed lines, accessory minerals; dotted lines, late or secondary minerals) and their compositional range. Mg-number = 100Mg/(Mg + Fe2+), where Mg and Fe2+ are expressed in number of cations per formula unit. Abbreviations as in Table 1, and Ilm, ilmenite; Tmt, titanomagnetite. UB., ultrabasic rocks.
Ultrabasic rocks
Fe–Mg-rich minerals represent more than 90% by volume in five samples, which are, therefore, ultrabasic in composition (Fig. 3). Samples THG-5C and -10B are mainly composed of a framework of sub-euhedral zoned diopside, with a grain size reaching 5 mm (Fig. 5a). Olivine crystals (≤3 mm) are euhedral. Fe–Mg KD values suggest that the olivines crystallized from a gabbroic liquid [equilibrium values of KD from Roeder & Emslie (1970)]. Their rims and cracks show secondary bowlingite alteration including titanomagnetite and chlorite (Fig. 5a). Inclusions of Cr-spinel have also been found. The other primary minerals present as interstitial phases are anhedral plagioclase (bytownite and labradorite, Fig. 6) with a length less than 2 mm, apatite, and Fe–Ti oxides (≤1 mm) displaying exsolution features and occasionally mantled by brown biotite. In the olivine–clinopyroxene–hornblende ternary diagram of Fig. 3, they plot in the olivine-clinopyroxenite field.
Photomicrographs of representative samples from Ahititera and Faaroa (plane-polarized light). (a) Olivine-clinopyroxenite THG-10B (Tahiti Nui). Mesocumulate. (Note the partial transformation of cumulus olivines into bowlingite.) (b) Clinopyroxene-hornblendite THG-14 (Tahiti Nui). Orthocumulate. The cumulus amphiboles contain various mineral inclusions. (c) Gabbro RIG-3B (Raiatea). Granular texture, close to the oikocrystic end-member. (d) Essexite THG-3A (Tahiti Nui). Intergranular texture, cumulus clinopyroxene and amphibole (these latter may result from cpx replacement). In the early stage of cpx transformation, the secondary hornblende (hastingsite or kaersutite in composition) occurs in the Fe-rich diopsidic margin, either as flakes associated with magnetite grains or as inclusion-free rims. Ap, apatite; Cpx, clinopyroxene; Hbl, hornblende; Ne, nepheline; Ol, olivine; Fe–Ti Ox, Fe–Ti oxides; Plg, plagioclase; Di, diopside; Kaer/Hast, kaersutite/hastingsite.
Classification of feldspars in the orthoclase–albite–anorthite ternary diagram.
The hornblendes of samples THG-2D, -14, and -7A are brown kaersutites (>1 cm, Fig. 5b). They include rare olivine, destabilized clinopyroxene, Fe–Ti oxides, and apatite. The amphibole crystals are usually euhedral (THG-14 and -7A), but occasionally subhedral and poikilitic (THG-2D). The rims of diopsidic clinopyroxenes are often partially replaced by brown kaersutite or hastingsite (Fig. 7a). Among the interstitial crystals, plagioclase (andesine, Fig. 6) is the most abundant phase. Others are sparse diopside, Fe–Ti oxides, large apatite (several hundreds of micrometres), nepheline, titanite, and K-feldspar. In the ternary diagram of Fig. 3, these samples plot in the clinopyroxene-hornblendite field.
Classification of Ahititera amphiboles. (a) Ti vs Mg-number (cations per formula unit) classification diagram (Leake et al., 1997). The cores of primary amphiboles plot within the kaersutite quadrant, whereas their rims and the replacement hornblendes extend towards the hastingsite–pargasite quadrant. (b) (AlIV + Ca) vs (Si + Na + K) variation diagram (expressed as cations per formula unit). There is a clear distinction between the early cores and the late amphiboles (rims and replacement minerals).
Nepheline-free rocks
In samples RIG-3B (Fig. 5c) and RI-28 from Raiatea, and THG-1B, -1 Da, -7B, -10C, and -10E from Tahiti, the most abundant minerals are sub-euhedral unzoned diopside (≤2 mm) and small plagioclase laths (from bytownite to K-bearing oligoclase in composition, Fig. 6). Large, hopper titanomagnetite and haemoilmenite crystals (<2 mm) are commonly bordered by brown or red biotite. Olivines are subordinate and their rims are usually mantled by radial green biotite plus oxides. The rest of the matrix is made up of primary biotite and apatite. In the layered rock THG-10E, the hydrous phase is amphibole instead of mica. Lack of K-feldspar, quartz, and foids in these rocks leads us to use the pyroxene–olivine–plagioclase diagram of Fig. 3 for classification, where they plot in the gabbro field.
Monzonites 37H and TP6, described by Bardintzeff et al. (1988), belong to the only petrographic type that was not recognized during our 1999 field sampling programme. They are made up mainly of Carlsbad-twinned alkali feldspar and plagioclase (Table 2). Giant amphiboles up to a few centimetres long, biotite, Fe–Ti oxides and titanite are their other mineral phases (Bardintzeff et al., 1988).
THG-9B, sampled in the hydrothermalized intrusion near the Vaituoru river dam (Fig. 2a), is an almost monomineralic rock formed of c. 83 wt % feldspar (albite and sparser orthoclase, Figs 4 and 6), accompanied by destabilized clinopyroxene, biotite, apatite, titanomagnetite, and especially abundant pyrite (≥1%). This mineral association is almost entirely secondary and evidences the occurrence of a hydrothermal process that affected the whole intrusion. THG-9B plots in the alkali syenite field in the APF diagram (Fig. 3).
Nepheline-bearing rocks
Samples RIG-1B, -1E, -2A, -2C, -2D, -4A, 4B, 4C, and RI-85, -86 (Raiatea), THG-1A, -2A, -2B, -9E2, and -13A (Tahiti), display a mineralogical association comparable with that of the gabbros, except for the presence of nepheline, more or less altered to cancrinite and analcite, rare titanite crystals, calcite, and, infrequently, late amphibole replacing clinopyroxene (Table 2 and Fig. 4). The two samples THG-1A and -2A are cumulative in olivine and/or zoned diopside (Table 2). These samples plot in the theralite field in the APF diagram of Fig. 3.
In samples THG-1E, -2C, -3A, -9E1, and -11A, the main Fe–Mg-rich crystals are brown amphibole (a few millimetres in diameter). They are either apatite-rich zoned euhedral kaersutites or late amphiboles, kaersutitic or hastingsitic in composition, replacing clinopyroxene rims (Table 3, Figs 5d and 7). The large clinopyroxene crystals (1–2 mm) are zoned: their chemical composition ranges from brown diopsidic cores to green ferro-diopsidic–hedenbergitic rims (Table 3). This latter composition is also recorded by small interstitial green clinopyroxenes. Olivines are sparse and altered, with the most magnesian composition of the entire set of olivine analyses (Fo 78–83, Fig. 4). This feature suggests they may be xenocrysts crystallized at the expense of a more mafic liquid. Plagioclase is still the main felsic phase, but its proportion is counterbalanced by that of K-feldspar plus nepheline (Table 2, Fig. 4). These three minerals are relatively altered: plagioclase and alkali feldspar are partially albitized, as shown in Fig. 6, and nepheline is partially transformed into albite plus analcite. Titanite is present as large euhedral crystals, up to 3 mm in length. Carbonates (ankerite and calcite) occur either in an interstitial position or as fillings of dictytaxitic voids. THG-3A and -11A are cumulative samples, mainly in clinopyroxene (Table 2). These samples plot in the essexite field (Fig. 3).
From essexites to samples THG-4, -6A, -1Db, -3B, and -19, the modal proportions of felsic minerals increase gradually, in contrast to those of Fe–Mg (plus Fe–Ti) minerals; alkali feldspar becomes progressively more abundant with respect to plagioclase (Table 2, Fig. 4). These samples are also characterized by the presence of destabilized titanite crystals and carbonates (calcite and ankerite). Nepheline and feldspars show alteration features similar to those observed in the essexites. Samples THG-4 and -6A are nepheline-monzosyenites whereas samples THG-1Db, -3B, and -19 plot in the nepheline-syenite field (Fig. 3).
Spatial distribution of the petrographic types
Tahiti Nui
Among the ultrabasic rocks, olivine-clinopyroxenites were collected from two distinct sites (Fig. 2a). The clinopyroxene-hornblendite group, not recognized prior to this study, does not exhibit clear field relationships with any other type of rock.
The nepheline-free group includes five gabbros, two monzonites, and one alkali syenite (Table 1, Figs 2a and 4). These nepheline-free rocks occur exclusively in the outer part of the outcropping area of the plutonic body, which is consistent with the results of the 1971 sampling reported by Bardintzeff et al. (1988).
The third petrographic group, constituted by nepheline-bearing rocks, is mainly found as large boulders coming from the at present unreachable central part of the pluton (Fig. 2a). It is composed of five theralites, five essexites, two nepheline-monzosyenites (not previously recognized), and three nepheline-syenites (Table 1; Fig. 3).
From a petrographic point of view, most of the Ahititera coarse-grained rocks can be regarded as the plutonic equivalents of the neighbouring lavas (ankaramites, alkali basalts, basanites and some intermediate lavas: Bonin & Bardintzeff, 1989; Clément et al., 2002).
Lobate contacts have been observed between olivine-clinopyroxenite THG-10B and gabbro THG-10C, and between essexite THG-3A and nepheline-syenite THG-3B (Table 1). Such associations demonstrate that both magmas were emplaced contemporaneously and they strongly suggest a petrogenetic link between them.
The schematic map of Fig. 2a summarizes our field observations and petrographic determinations, together with those made by Nitecki-Novotny (1975) and Bardintzeff et al. (1988). Our map displays some major differences compared with that of Bardintzeff et al. (1988). Both of them are in good agreement regarding the concentric zonation of the pluton (nepheline-bearing rocks in its central part and nepheline-free rocks at its periphery). However, we did not find evidence for vertical layering at the pluton scale: some petrographic types such as essexites and ol-clinopyroxenites crop out at equivalent heights above sea level. The Ahititera pluton seems to be mostly made up of two nested intrusions. The very slow crystallization of the plutonic body and its local alteration preclude any dating on coarse-grained samples. However, the presence of nepheline-bearing rocks in the inner part of the body, together with the additional geochemical arguments developed by Clément et al. (2002), suggest a late emplacement of these rocks with respect to the nepheline-free ones. Finally, as previously shown by Clément et al. (2002), a large area of the plutonic massif is sealed by a thick epiclastic formation.
Raiatea
Only two petrographic types have been identified and are located on the Fig. 2b map: two nepheline-free gabbros and 10 nepheline-bearing theralites (Table 1, Fig. 3). The gabbros were sampled in the bed of the southern tributary of Apoomau river. The theralites are exposed in three areas: the northern tributary of Apoomau river, on the flank of a 57 m high hill located between the two Apoomau tributaries, and on a 30 m high hill located near the crossroad of the island. The poor outcrop conditions in the Faaroa depression have prevented more precise mapping of the pluton.
GEOCHEMISTRY
Inductively coupled plasma atomic emission spectrometry (ICP-AES) analyses of 40 samples from Tahiti and Raiatea are presented in Table 4. The analytical method has been described by Cotten et al. (1995). Relative standard deviations are <2% for major elements, Rb and Sr, and <5% for other trace elements. Sr and Nd isotopic analyses have been performed following the procedure described by Dosso et al. (1991). To remove alteration effects, the whole-rock powders were leached with 2·5N HCl for 10 min in an ultrasonic bath and rinsed three times in ultrapure water. Sr isotope ratios were measured with a Finnigan MAT 261 mass spectrometer (IFREMER, Brest) in dynamic mode. Sr isotope compositions are corrected for mass fractionation to 88Sr/86Sr = 8·375209 and referenced to NBS SRM987 = 0·710247 ± 0·00012 (n = 30). Nd ratios have been measured with a ThermoFinnigan Triton T1 mass spectrometer (IUEM, Brest) in static mode. Nd isotope compositions are corrected for mass fractionation to 146Nd/144Nd = 0·721903 and referenced to La Jolla-Nd = 0·511850 ± 0·000007 (n = 65) and to JNd1 = 0·512107 ± 0·000008 (n = 35).
Major and trace elements, and Nd–Sr isotopes from Ahititera (Tahiti Nui) and Faaroa (Raiatea) samples
| Island: . | Tahiti . | Tahiti . | Tahiti . | Tahiti . | Tahiti . | Tahiti . | Tahiti . | Tahiti . | Tahiti . | Tahiti . | Tahiti . | Tahiti . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample: . | THG-10B . | THG-5C . | THG-10C . | THG-10E . | THG-1B . | THG-1 Da . | THG-7B . | TP6 . | 37H . | THG-9B . | THG-2D . | THG-14 . |
| Type: . | Ol-Cpxite . | Ol-Cpxite . | Gab . | Gab . | Gab . | Gab . | Gab . | Monz . | Monz . | Alk-Sye . | Cpx-Hblite . | Cpx-Hblite . |
| Group: . | 2 . | 2 . | 2 . | 2 . | 2 . | 2 . | 2 . | 2 . | 2 . | 2 . | 1 . | 1 . |
| Reference: . | a . | a . | b . | a . | a . | a . | a . | c . | d . | a . | a . | a . |
| SiO2 | 41·40 | 43·80 | 45·30 | 40·40 | 44·00 | 42·80 | 43·00 | 46·30 | 49·23 | 61·00 | 36·00 | 38·60 |
| TiO2 | 1·90 | 2·52 | 3·36 | 6·60 | 5·35 | 4·10 | 4·52 | 4·27 | 3·33 | 0·65 | 5·45 | 4·59 |
| Al2O3 | 6·08 | 9·65 | 13·90 | 13·70 | 15·00 | 16·10 | 13·72 | 17·97 | 17·24 | 18·40 | 14·35 | 11·70 |
| Fe2O3t | 14·70 | 12·45 | 14·00 | 13·45 | 13·70 | 14·20 | 15·60 | 11·95 | 10·30 | 4·90 | 15·60 | 15·60 |
| MnO | 0·20 | 0·19 | 0·15 | 0·16 | 0·17 | 0·17 | 0·18 | 0·19 | 0·16 | 0·03 | 0·21 | 0·22 |
| MgO | 20·30 | 13·20 | 6·47 | 8·04 | 6·02 | 5·60 | 6·55 | 2·77 | 4·26 | 0·47 | 7·95 | 9·50 |
| CaO | 10·50 | 14·65 | 11·35 | 14·55 | 10·50 | 11·90 | 12·25 | 7·62 | 7·13 | 0·37 | 14·40 | 13·85 |
| Na2O | 0·52 | 1·06 | 3·12 | 1·76 | 3·00 | 2·70 | 2·32 | 3·03 | 4·41 | 7·30 | 2·43 | 2·35 |
| K2O | 0·53 | 0·41 | 1·13 | 0·56 | 1·78 | 1·08 | 0·90 | 2·41 | 1·88 | 4·90 | 1·22 | 1·45 |
| P2O5 | 0·20 | 0·15 | 0·46 | 0·17 | 0·40 | 0·32 | 0·42 | 0·96 | n.d. | 0·13 | 1·90 | 0·83 |
| LOI | 3·25 | 1·68 | 0·79 | 0·42 | 0·44 | 0·15 | 0·45 | 1·81 | 1·99 | 1·51 | 0·35 | 0·91 |
| Total | 99·58 | 99·76 | 100·03 | 99·81 | 100·36 | 99·12 | 99·91 | 99·28 | 99·93 | 99·66 | 99·86 | 99·60 |
| Q | 0·5 | |||||||||||
| Hy | 9·8 | 1·7 | ||||||||||
| Ne + Lc | 0·3 | 2·3 | 5·2 | 6·5 | 6·2 | 6·4 | 3·8 | 0·0 | 0·0 | 1·4 | 16·8 | 17·5 |
| Rb | 12·0 | 8·2 | 26·0 | 18·0 | 40·5 | 22·5 | 19·0 | 75 | 61·7 | 104·0 | 20·5 | 40·0 |
| Sr | 166 | 424 | 700 | 750 | 795 | 910 | 810 | 1134 | 858 | 270 | 1390 | 720 |
| Ba | 75 | 185 | 360 | 142 | 360 | 33 | 320 | 692 | 868 | 1130 | 650 | 540 |
| Sc | 34·0 | 45·0 | 28·0 | 35·5 | 24·0 | 21·0 | 26·0 | n.d. | 722·0 | 0·6 | 14·0 | 26·0 |
| V | 240 | 310 | 420 | 425 | 419 | 480 | 560 | n.d. | 254 | 8 | 440 | 460 |
| Cr | 1260 | 900 | 80 | 175 | 78 | 46 | 85 | 12 | 127 | 4 | 41 | 209 |
| Co | 86 | 59 | 51 | 47 | 46 | 51 | 52 | 44 | 18 | 2 | 41 | 54 |
| Ni | 660 | 300 | 165 | 130 | 92 | 110 | 115 | 54 | 7 | 4 | 50 | 145 |
| Y | 14·0 | 19·3 | 28·0 | 20·8 | 24·0 | 22·0 | 27·5 | n.d. | n.d. | 21·5 | 49·0 | 38·5 |
| Zr | 68 | 137 | 175 | 223 | 270 | 215 | 258 | n.d. | n.d. | 420 | 235 | 300 |
| Nb | 11·6 | 11·0 | 37·0 | 30·5 | 47·0 | 34·0 | 32·0 | n.d. | n.d. | 83·0 | 55·0 | 57·0 |
| La | 11·0 | 12·6 | 31·0 | 18·0 | 33·5 | 25·0 | 29·5 | n.d. | 85·3 | 78·0 | 60·0 | 46·0 |
| Ce | 27·0 | 34·0 | 70·0 | 42·5 | 74·0 | 57·0 | 70·0 | n.d. | 130·0 | 148·0 | 142·0 | 106·0 |
| Nd | 18·0 | 24·0 | 42·0 | 29·5 | 42·0 | 34·0 | 44·0 | n.d. | n.d. | 58·0 | 92·0 | 62·0 |
| Sm | 4·60 | 6·05 | 8·90 | 6·90 | 8·40 | 7·15 | 9·05 | n.d. | 10·70 | 9·10 | 17·60 | 12·90 |
| Eu | 1·30 | 1·95 | 2·62 | 2·07 | 2·48 | 2·45 | 2·89 | n.d. | 5·00 | 2·54 | 5·37 | 3·73 |
| Gd | 3·75 | 5·80 | 7·90 | 6·35 | 7·50 | 6·20 | 8·00 | n.d. | n.d. | 6·60 | 16·00 | 11·00 |
| Dy | 3·10 | 4·30 | 5·90 | 4·50 | 5·20 | 4·60 | 6·00 | n.d. | 7·10 | 3·90 | 10·40 | 8·00 |
| Er | 1·40 | 1·70 | 2·60 | 1·80 | 2·10 | 1·90 | 2·50 | n.d. | n.d. | 1·80 | 4·10 | 3·40 |
| Yb | 1·02 | 1·20 | 1·90 | 1·34 | 1·58 | 1·25 | 1·71 | n.d. | 1·12 | 1·75 | 2·72 | 2·36 |
| Th | 0·80 | 0·85 | 3·30 | 2·30 | 4·30 | 2·90 | 3·05 | n.d. | 7·44 | 11·20 | 2·80 | 3·05 |
| 87Sr/86Sr | 0·704566 ± 10 | 0·704659 ± 9 | 0·704532 ± 9 | 0·703964 ± 10 | ||||||||
| 143Nd/144Nd | 0·512774 ± 1 | 0·512769 ± 7 | 0·512782 ± 3 | 0·512893 ± 2 |
| Island: . | Tahiti . | Tahiti . | Tahiti . | Tahiti . | Tahiti . | Tahiti . | Tahiti . | Tahiti . | Tahiti . | Tahiti . | Tahiti . | Tahiti . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample: . | THG-10B . | THG-5C . | THG-10C . | THG-10E . | THG-1B . | THG-1 Da . | THG-7B . | TP6 . | 37H . | THG-9B . | THG-2D . | THG-14 . |
| Type: . | Ol-Cpxite . | Ol-Cpxite . | Gab . | Gab . | Gab . | Gab . | Gab . | Monz . | Monz . | Alk-Sye . | Cpx-Hblite . | Cpx-Hblite . |
| Group: . | 2 . | 2 . | 2 . | 2 . | 2 . | 2 . | 2 . | 2 . | 2 . | 2 . | 1 . | 1 . |
| Reference: . | a . | a . | b . | a . | a . | a . | a . | c . | d . | a . | a . | a . |
| SiO2 | 41·40 | 43·80 | 45·30 | 40·40 | 44·00 | 42·80 | 43·00 | 46·30 | 49·23 | 61·00 | 36·00 | 38·60 |
| TiO2 | 1·90 | 2·52 | 3·36 | 6·60 | 5·35 | 4·10 | 4·52 | 4·27 | 3·33 | 0·65 | 5·45 | 4·59 |
| Al2O3 | 6·08 | 9·65 | 13·90 | 13·70 | 15·00 | 16·10 | 13·72 | 17·97 | 17·24 | 18·40 | 14·35 | 11·70 |
| Fe2O3t | 14·70 | 12·45 | 14·00 | 13·45 | 13·70 | 14·20 | 15·60 | 11·95 | 10·30 | 4·90 | 15·60 | 15·60 |
| MnO | 0·20 | 0·19 | 0·15 | 0·16 | 0·17 | 0·17 | 0·18 | 0·19 | 0·16 | 0·03 | 0·21 | 0·22 |
| MgO | 20·30 | 13·20 | 6·47 | 8·04 | 6·02 | 5·60 | 6·55 | 2·77 | 4·26 | 0·47 | 7·95 | 9·50 |
| CaO | 10·50 | 14·65 | 11·35 | 14·55 | 10·50 | 11·90 | 12·25 | 7·62 | 7·13 | 0·37 | 14·40 | 13·85 |
| Na2O | 0·52 | 1·06 | 3·12 | 1·76 | 3·00 | 2·70 | 2·32 | 3·03 | 4·41 | 7·30 | 2·43 | 2·35 |
| K2O | 0·53 | 0·41 | 1·13 | 0·56 | 1·78 | 1·08 | 0·90 | 2·41 | 1·88 | 4·90 | 1·22 | 1·45 |
| P2O5 | 0·20 | 0·15 | 0·46 | 0·17 | 0·40 | 0·32 | 0·42 | 0·96 | n.d. | 0·13 | 1·90 | 0·83 |
| LOI | 3·25 | 1·68 | 0·79 | 0·42 | 0·44 | 0·15 | 0·45 | 1·81 | 1·99 | 1·51 | 0·35 | 0·91 |
| Total | 99·58 | 99·76 | 100·03 | 99·81 | 100·36 | 99·12 | 99·91 | 99·28 | 99·93 | 99·66 | 99·86 | 99·60 |
| Q | 0·5 | |||||||||||
| Hy | 9·8 | 1·7 | ||||||||||
| Ne + Lc | 0·3 | 2·3 | 5·2 | 6·5 | 6·2 | 6·4 | 3·8 | 0·0 | 0·0 | 1·4 | 16·8 | 17·5 |
| Rb | 12·0 | 8·2 | 26·0 | 18·0 | 40·5 | 22·5 | 19·0 | 75 | 61·7 | 104·0 | 20·5 | 40·0 |
| Sr | 166 | 424 | 700 | 750 | 795 | 910 | 810 | 1134 | 858 | 270 | 1390 | 720 |
| Ba | 75 | 185 | 360 | 142 | 360 | 33 | 320 | 692 | 868 | 1130 | 650 | 540 |
| Sc | 34·0 | 45·0 | 28·0 | 35·5 | 24·0 | 21·0 | 26·0 | n.d. | 722·0 | 0·6 | 14·0 | 26·0 |
| V | 240 | 310 | 420 | 425 | 419 | 480 | 560 | n.d. | 254 | 8 | 440 | 460 |
| Cr | 1260 | 900 | 80 | 175 | 78 | 46 | 85 | 12 | 127 | 4 | 41 | 209 |
| Co | 86 | 59 | 51 | 47 | 46 | 51 | 52 | 44 | 18 | 2 | 41 | 54 |
| Ni | 660 | 300 | 165 | 130 | 92 | 110 | 115 | 54 | 7 | 4 | 50 | 145 |
| Y | 14·0 | 19·3 | 28·0 | 20·8 | 24·0 | 22·0 | 27·5 | n.d. | n.d. | 21·5 | 49·0 | 38·5 |
| Zr | 68 | 137 | 175 | 223 | 270 | 215 | 258 | n.d. | n.d. | 420 | 235 | 300 |
| Nb | 11·6 | 11·0 | 37·0 | 30·5 | 47·0 | 34·0 | 32·0 | n.d. | n.d. | 83·0 | 55·0 | 57·0 |
| La | 11·0 | 12·6 | 31·0 | 18·0 | 33·5 | 25·0 | 29·5 | n.d. | 85·3 | 78·0 | 60·0 | 46·0 |
| Ce | 27·0 | 34·0 | 70·0 | 42·5 | 74·0 | 57·0 | 70·0 | n.d. | 130·0 | 148·0 | 142·0 | 106·0 |
| Nd | 18·0 | 24·0 | 42·0 | 29·5 | 42·0 | 34·0 | 44·0 | n.d. | n.d. | 58·0 | 92·0 | 62·0 |
| Sm | 4·60 | 6·05 | 8·90 | 6·90 | 8·40 | 7·15 | 9·05 | n.d. | 10·70 | 9·10 | 17·60 | 12·90 |
| Eu | 1·30 | 1·95 | 2·62 | 2·07 | 2·48 | 2·45 | 2·89 | n.d. | 5·00 | 2·54 | 5·37 | 3·73 |
| Gd | 3·75 | 5·80 | 7·90 | 6·35 | 7·50 | 6·20 | 8·00 | n.d. | n.d. | 6·60 | 16·00 | 11·00 |
| Dy | 3·10 | 4·30 | 5·90 | 4·50 | 5·20 | 4·60 | 6·00 | n.d. | 7·10 | 3·90 | 10·40 | 8·00 |
| Er | 1·40 | 1·70 | 2·60 | 1·80 | 2·10 | 1·90 | 2·50 | n.d. | n.d. | 1·80 | 4·10 | 3·40 |
| Yb | 1·02 | 1·20 | 1·90 | 1·34 | 1·58 | 1·25 | 1·71 | n.d. | 1·12 | 1·75 | 2·72 | 2·36 |
| Th | 0·80 | 0·85 | 3·30 | 2·30 | 4·30 | 2·90 | 3·05 | n.d. | 7·44 | 11·20 | 2·80 | 3·05 |
| 87Sr/86Sr | 0·704566 ± 10 | 0·704659 ± 9 | 0·704532 ± 9 | 0·703964 ± 10 | ||||||||
| 143Nd/144Nd | 0·512774 ± 1 | 0·512769 ± 7 | 0·512782 ± 3 | 0·512893 ± 2 |
| Island: . | Tahiti . | Tahiti . | Tahiti . | Tahiti . | Tahiti . | Tahiti . | Tahiti . | Tahiti . | Tahiti . | Tahiti . | Tahiti . | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample: . | THG-7A . | THG-13A . | THG-9E2 . | THG-2B . | THG-2A . | THG-2A* . | THG-1A . | THG-1A* . | THG-2C . | THG-9E1 . | THG-1E . | |||||||||||
| Type: . | Cpx-Hblite . | The . | The . | The . | The . | The . | The . | The . | Ess . | Ess . | Ess . | |||||||||||
| Group: . | 1 . | 1 . | 1 . | 3 . | 3 . | 3 . | 3 . | 3 . | 1 . | 1 . | 1 . | |||||||||||
| Reference: . | a . | b . | a . | b . | a . | . | a . | . | a . | b . | a . | |||||||||||
| SiO2 | 37·30 | 44·70 | 43·40 | 46·50 | 45·30 | 46·06 | 45·00 | 46·95 | 44·70 | 45·50 | 44·50 | |||||||||||
| TiO2 | 5·35 | 3·88 | 3·33 | 3·85 | 3·30 | 4·21 | 2·70 | 3·93 | 2·85 | 2·75 | 3·23 | |||||||||||
| Al2O3 | 12·45 | 15·85 | 19·80 | 17·35 | 13·05 | 17·40 | 11·30 | 17·49 | 18·45 | 18·00 | 17·20 | |||||||||||
| Fe2O3t | 17·15 | 12·60 | 12·05 | 11·30 | 12·64 | 11·28 | 13·60 | 11·34 | 9·75 | 9·60 | 10·63 | |||||||||||
| MnO | 0·22 | 0·20 | 0·19 | 0·14 | 0·16 | 0·13 | 0·17 | 0·13 | 0·20 | 0·20 | 0·20 | |||||||||||
| MgO | 8·90 | 4·92 | 3·60 | 3·50 | 9·70 | 3·57 | 13·35 | 3·47 | 3·94 | 3·95 | 4·15 | |||||||||||
| CaO | 12·60 | 9·20 | 9·48 | 9·20 | 9·80 | 9·31 | 8·86 | 9·14 | 8·38 | 8·20 | 8·80 | |||||||||||
| Na2O | 2·28 | 4·30 | 4·47 | 4·40 | 2·93 | 3·90 | 2·53 | 3·91 | 5·30 | 5·25 | 5·80 | |||||||||||
| K2O | 1·46 | 1·85 | 2·02 | 2·40 | 2·10 | 2·91 | 1·39 | 2·25 | 3·46 | 3·70 | 3·50 | |||||||||||
| P2O5 | 0·92 | 0·80 | 0·87 | 0·66 | 0·46 | 0·63 | 0·46 | 0·74 | 0·95 | 0·92 | 0·78 | |||||||||||
| LOI | 1·04 | 1·53 | 0·48 | 0·39 | 0·48 | 0·48 | 0·23 | 0·23 | 2·24 | 1·94 | 1·13 | |||||||||||
| Total | 99·67 | 99·83 | 99·69 | 99·69 | 99·92 | 99·92 | 99·59 | 99·59 | 100·22 | 100·01 | 99·92 | |||||||||||
| Q | ||||||||||||||||||||||
| Hy | ||||||||||||||||||||||
| Ne + Lc | 17·2 | 10·0 | 13·9 | 9·9 | 7·2 | 9·5 | 3·5 | 5·4 | 19·3 | 18·6 | 23·8 | |||||||||||
| Rb | 37·5 | 49·0 | 51·0 | 56·0 | 44·5 | 60·6 | 27·5 | 42·5 | 115·0 | 102·0 | 103·0 | |||||||||||
| Sr | 850 | 1000 | 1590 | 1080 | 750 | 1013 | 560 | 866 | 1470 | 1380 | 1030 | |||||||||||
| Ba | 660 | 730 | 760 | 680 | 515 | 706 | 380 | 595 | 1070 | 1200 | 915 | |||||||||||
| Sc | 23·0 | 17·0 | 6·5 | 11·0 | 22·0 | 22·5 | 2·7 | 4·0 | 10·0 | |||||||||||||
| V | 500 | 310 | 282 | 242 | 270 | 230 | 215 | 195 | 262 | |||||||||||||
| Cr | 170 | 59 | 12 | 10 | 480 | 580 | 1 | 1 | 6 | |||||||||||||
| Co | 55 | 36 | 31 | 29 | 50 | 64 | 17 | 21 | 24 | |||||||||||||
| Ni | 120 | 72 | 21 | 50 | 238 | 407 | 1 | 2 | 17 | |||||||||||||
| Y | 38·0 | 36·0 | 28·0 | 34·0 | 26·5 | 32·5 | 22·0 | 30·3 | 39·0 | 34·0 | 37·5 | |||||||||||
| Zr | 270 | 330 | 255 | 365 | 278 | 215 | 382 | 296 | 405 | |||||||||||||
| Nb | 57·0 | 75·0 | 61·0 | 60·0 | 44·0 | 60·7 | 34·0 | 53·9 | 93·0 | 81·0 | 93·0 | |||||||||||
| La | 42·0 | 57·0 | 54·0 | 64·0 | 45·0 | 61·3 | 34·5 | 53·7 | 78·0 | 70·0 | 69·5 | |||||||||||
| Ce | 100·0 | 120·0 | 109·0 | 128·0 | 96·0 | 128·7 | 74·0 | 113·5 | 165·0 | 142·0 | 140·0 | |||||||||||
| Nd | 66·0 | 60·5 | 53·0 | 65·0 | 50·0 | 62·4 | 41·0 | 57·7 | 79·0 | 67·0 | 70·0 | |||||||||||
| Sm | 13·00 | 11·80 | 9·75 | 11·80 | 9·60 | 11·98 | 8·20 | 11·54 | 13·80 | 11·60 | 12·20 | |||||||||||
| Eu | 3·95 | 3·53 | 3·21 | 3·67 | 2·83 | 3·53 | 2·35 | 3·29 | 3·95 | 3·38 | 3·60 | |||||||||||
| Gd | 11·40 | 10·20 | 8·10 | 10·20 | 8·20 | 10·05 | 7·00 | 9·65 | 11·00 | 9·75 | 10·50 | |||||||||||
| Dy | 8·30 | 7·50 | 5·80 | 7·00 | 5·70 | 6·99 | 4·90 | 6·69 | 8·00 | 6·80 | 7·60 | |||||||||||
| Er | 3·40 | 3·20 | 2·55 | 2·90 | 2·40 | 2·94 | 2·00 | 2·76 | 3·40 | 3·10 | 3·30 | |||||||||||
| Yb | 2·25 | 2·44 | 2·03 | 2·03 | 1·67 | 2·05 | 1·38 | 1·90 | 2·67 | 2·35 | 2·61 | |||||||||||
| Th | 3·15 | 7·45 | 6·10 | 8·80 | 5·90 | 8·11 | 4·45 | 6·99 | 10·40 | 6·80 | 9·15 | |||||||||||
| 87Sr/86Sr | 0·704147 ± 10 | 0·705257 ± 9 | 0·705143 ± 8 | 0·703963 ± 6 | 0·703971 ± 9 | |||||||||||||||||
| 143Nd/144Nd | 0·512884 ± 8 | 0·512718 ± 2 | 0·512729 ± 2 | 0·512883 ± 2 | 0·512892 ± 10 | |||||||||||||||||
| Island: . | Tahiti . | Tahiti . | Tahiti . | Tahiti . | Tahiti . | Tahiti . | Tahiti . | Tahiti . | Tahiti . | Tahiti . | Tahiti . | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample: . | THG-7A . | THG-13A . | THG-9E2 . | THG-2B . | THG-2A . | THG-2A* . | THG-1A . | THG-1A* . | THG-2C . | THG-9E1 . | THG-1E . | |||||||||||
| Type: . | Cpx-Hblite . | The . | The . | The . | The . | The . | The . | The . | Ess . | Ess . | Ess . | |||||||||||
| Group: . | 1 . | 1 . | 1 . | 3 . | 3 . | 3 . | 3 . | 3 . | 1 . | 1 . | 1 . | |||||||||||
| Reference: . | a . | b . | a . | b . | a . | . | a . | . | a . | b . | a . | |||||||||||
| SiO2 | 37·30 | 44·70 | 43·40 | 46·50 | 45·30 | 46·06 | 45·00 | 46·95 | 44·70 | 45·50 | 44·50 | |||||||||||
| TiO2 | 5·35 | 3·88 | 3·33 | 3·85 | 3·30 | 4·21 | 2·70 | 3·93 | 2·85 | 2·75 | 3·23 | |||||||||||
| Al2O3 | 12·45 | 15·85 | 19·80 | 17·35 | 13·05 | 17·40 | 11·30 | 17·49 | 18·45 | 18·00 | 17·20 | |||||||||||
| Fe2O3t | 17·15 | 12·60 | 12·05 | 11·30 | 12·64 | 11·28 | 13·60 | 11·34 | 9·75 | 9·60 | 10·63 | |||||||||||
| MnO | 0·22 | 0·20 | 0·19 | 0·14 | 0·16 | 0·13 | 0·17 | 0·13 | 0·20 | 0·20 | 0·20 | |||||||||||
| MgO | 8·90 | 4·92 | 3·60 | 3·50 | 9·70 | 3·57 | 13·35 | 3·47 | 3·94 | 3·95 | 4·15 | |||||||||||
| CaO | 12·60 | 9·20 | 9·48 | 9·20 | 9·80 | 9·31 | 8·86 | 9·14 | 8·38 | 8·20 | 8·80 | |||||||||||
| Na2O | 2·28 | 4·30 | 4·47 | 4·40 | 2·93 | 3·90 | 2·53 | 3·91 | 5·30 | 5·25 | 5·80 | |||||||||||
| K2O | 1·46 | 1·85 | 2·02 | 2·40 | 2·10 | 2·91 | 1·39 | 2·25 | 3·46 | 3·70 | 3·50 | |||||||||||
| P2O5 | 0·92 | 0·80 | 0·87 | 0·66 | 0·46 | 0·63 | 0·46 | 0·74 | 0·95 | 0·92 | 0·78 | |||||||||||
| LOI | 1·04 | 1·53 | 0·48 | 0·39 | 0·48 | 0·48 | 0·23 | 0·23 | 2·24 | 1·94 | 1·13 | |||||||||||
| Total | 99·67 | 99·83 | 99·69 | 99·69 | 99·92 | 99·92 | 99·59 | 99·59 | 100·22 | 100·01 | 99·92 | |||||||||||
| Q | ||||||||||||||||||||||
| Hy | ||||||||||||||||||||||
| Ne + Lc | 17·2 | 10·0 | 13·9 | 9·9 | 7·2 | 9·5 | 3·5 | 5·4 | 19·3 | 18·6 | 23·8 | |||||||||||
| Rb | 37·5 | 49·0 | 51·0 | 56·0 | 44·5 | 60·6 | 27·5 | 42·5 | 115·0 | 102·0 | 103·0 | |||||||||||
| Sr | 850 | 1000 | 1590 | 1080 | 750 | 1013 | 560 | 866 | 1470 | 1380 | 1030 | |||||||||||
| Ba | 660 | 730 | 760 | 680 | 515 | 706 | 380 | 595 | 1070 | 1200 | 915 | |||||||||||
| Sc | 23·0 | 17·0 | 6·5 | 11·0 | 22·0 | 22·5 | 2·7 | 4·0 | 10·0 | |||||||||||||
| V | 500 | 310 | 282 | 242 | 270 | 230 | 215 | 195 | 262 | |||||||||||||
| Cr | 170 | 59 | 12 | 10 | 480 | 580 | 1 | 1 | 6 | |||||||||||||
| Co | 55 | 36 | 31 | 29 | 50 | 64 | 17 | 21 | 24 | |||||||||||||
| Ni | 120 | 72 | 21 | 50 | 238 | 407 | 1 | 2 | 17 | |||||||||||||
| Y | 38·0 | 36·0 | 28·0 | 34·0 | 26·5 | 32·5 | 22·0 | 30·3 | 39·0 | 34·0 | 37·5 | |||||||||||
| Zr | 270 | 330 | 255 | 365 | 278 | 215 | 382 | 296 | 405 | |||||||||||||
| Nb | 57·0 | 75·0 | 61·0 | 60·0 | 44·0 | 60·7 | 34·0 | 53·9 | 93·0 | 81·0 | 93·0 | |||||||||||
| La | 42·0 | 57·0 | 54·0 | 64·0 | 45·0 | 61·3 | 34·5 | 53·7 | 78·0 | 70·0 | 69·5 | |||||||||||
| Ce | 100·0 | 120·0 | 109·0 | 128·0 | 96·0 | 128·7 | 74·0 | 113·5 | 165·0 | 142·0 | 140·0 | |||||||||||
| Nd | 66·0 | 60·5 | 53·0 | 65·0 | 50·0 | 62·4 | 41·0 | 57·7 | 79·0 | 67·0 | 70·0 | |||||||||||
| Sm | 13·00 | 11·80 | 9·75 | 11·80 | 9·60 | 11·98 | 8·20 | 11·54 | 13·80 | 11·60 | 12·20 | |||||||||||
| Eu | 3·95 | 3·53 | 3·21 | 3·67 | 2·83 | 3·53 | 2·35 | 3·29 | 3·95 | 3·38 | 3·60 | |||||||||||
| Gd | 11·40 | 10·20 | 8·10 | 10·20 | 8·20 | 10·05 | 7·00 | 9·65 | 11·00 | 9·75 | 10·50 | |||||||||||
| Dy | 8·30 | 7·50 | 5·80 | 7·00 | 5·70 | 6·99 | 4·90 | 6·69 | 8·00 | 6·80 | 7·60 | |||||||||||
| Er | 3·40 | 3·20 | 2·55 | 2·90 | 2·40 | 2·94 | 2·00 | 2·76 | 3·40 | 3·10 | 3·30 | |||||||||||
| Yb | 2·25 | 2·44 | 2·03 | 2·03 | 1·67 | 2·05 | 1·38 | 1·90 | 2·67 | 2·35 | 2·61 | |||||||||||
| Th | 3·15 | 7·45 | 6·10 | 8·80 | 5·90 | 8·11 | 4·45 | 6·99 | 10·40 | 6·80 | 9·15 | |||||||||||
| 87Sr/86Sr | 0·704147 ± 10 | 0·705257 ± 9 | 0·705143 ± 8 | 0·703963 ± 6 | 0·703971 ± 9 | |||||||||||||||||
| 143Nd/144Nd | 0·512884 ± 8 | 0·512718 ± 2 | 0·512729 ± 2 | 0·512883 ± 2 | 0·512892 ± 10 | |||||||||||||||||
| Island: . | Tahiti . | Tahiti . | Tahiti . | Tahiti . | Tahiti . | Tahiti . | Tahiti . | Tahiti . | Tahiti . | Raiatea . | Raiatea . | Raiatea . | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample: . | THG-11A . | THG-11A* . | THG-3A . | THG-3A* . | THG-4 . | THG-6A . | THG-3B . | THG-19 . | THG-1Db . | RIG-3B . | RI-28 . | RIG-1B . | ||||||||||||
| Type: . | Ess . | Ess . | Ess . | Ess . | Ne-Msye . | Ne-Msye . | Ne-Sye . | Ne-Sye . | Ne-Sye . | Gab . | Gab . | The . | ||||||||||||
| Group: . | 1 . | 1 . | 1 . | 1 . | 1 . | 1 . | 1 . | 1 . | 1 . | . | . | . | ||||||||||||
| Reference: . | a . | . | a . | . | a . | a . | a . | a . | a . | a . | a, e . | a . | ||||||||||||
| SiO2 | 42·40 | 44·43 | 42·30 | 44·74 | 49·00 | 48·00 | 50·70 | 52·30 | 54·00 | 44·30 | 44·50 | 47·20 | ||||||||||||
| TiO2 | 3·59 | 2·79 | 3·65 | 2·81 | 2·01 | 2·29 | 1·55 | 1·15 | 1·42 | 6·30 | 6·05 | 3·04 | ||||||||||||
| Al2O3 | 15·35 | 18·52 | 15·00 | 18·99 | 19·05 | 18·60 | 20·45 | 20·30 | 18·60 | 15·00 | 14·43 | 18·50 | ||||||||||||
| Fe2O3t | 12·70 | 9·74 | 13·10 | 9·81 | 7·42 | 8·85 | 5·42 | 4·48 | 6·90 | 12·40 | 12·77 | 10·45 | ||||||||||||
| MnO | 0·20 | 0·11 | 0·22 | 0·12 | 0·16 | 0·19 | 0·13 | 0·16 | 0·12 | 0·15 | 0·15 | 0·15 | ||||||||||||
| MgO | 5·88 | 4·15 | 6·25 | 4·19 | 2·78 | 3·05 | 1·22 | 0·89 | 1·21 | 12·00 | 11·50 | 8·10 | ||||||||||||
| CaO | 10·70 | 8·54 | 11·30 | 8·49 | 6·13 | 6·45 | 4·95 | 3·70 | 3·12 | 12·00 | 11·50 | 8·10 | ||||||||||||
| Na2O | 4·45 | 5·81 | 3·90 | 5·46 | 6·40 | 6·20 | 5·95 | 7·00 | 6·52 | 2·80 | 2·82 | 5·00 | ||||||||||||
| K2O | 2·63 | 3·52 | 1·84 | 2·67 | 3·88 | 3·95 | 4·88 | 6·10 | 6·70 | 1·05 | 1·18 | 3·10 | ||||||||||||
| P2O5 | 0·85 | 1·14 | 0·64 | 0·93 | 0·56 | 0·53 | 0·28 | 0·21 | 0·34 | 0·38 | 0·40 | 1·00 | ||||||||||||
| LOI | 1·08 | 1·08 | 1·20 | 1·20 | 2·37 | 1·80 | 4·34 | 3·63 | 1·09 | 0·25 | 0·32 | 0·44 | ||||||||||||
| Total | 99·83 | 99·83 | 99·40 | 99·40 | 99·76 | 99·91 | 99·87 | 99·92 | 100·02 | 100·23 | 99·86 | 100·03 | ||||||||||||
| Q | ||||||||||||||||||||||||
| Hy | ||||||||||||||||||||||||
| Ne + Lc | 18·8 | 23·1 | 14·1 | 17·6 | 18·6 | 19·8 | 14·7 | 21·5 | 17·3 | 3·2 | 2·8 | 12·9 | ||||||||||||
| Rb | 75·5 | 99·3 | 44·0 | 61·5 | 121·0 | 123·0 | 152·0 | 205·0 | 155·0 | 20·5 | 26·0 | 73·0 | ||||||||||||
| Sr | 905 | 1157 | 1035 | 1391 | 1020 | 1010 | 1465 | 890 | 495 | 870 | 800 | 1230 | ||||||||||||
| Ba | 740 | 979 | 720 | 1013 | 1140 | 1050 | 1350 | 1270 | 1050 | 285 | 302 | 740 | ||||||||||||
| Sc | 17·8 | 21·0 | 5·8 | 6·8 | 0·8 | 0·4 | 2·5 | 25·0 | 25·5 | 6·0 | ||||||||||||||
| V | 333 | 320 | 145 | 158 | 82 | 55 | 28 | 425 | 430 | 140 | ||||||||||||||
| Cr | 63 | 102 | 30 | 26 | 1 | 3 | 2 | 54 | 65 | 7 | ||||||||||||||
| Co | 39 | 40 | 17 | 21 | 6 | 4 | 9 | 40 | 42 | 25 | ||||||||||||||
| Ni | 55 | 61 | 17 | 22 | 2 | 2 | 4 | 95 | 97 | 25 | ||||||||||||||
| Y | 34·8 | 38·6 | 34·0 | 38·1 | 28·5 | 30·0 | 32·0 | 26·0 | 46·0 | 22·5 | 25·0 | 36·5 | ||||||||||||
| Zr | 350 | 315 | 280 | 270 | 335 | 495 | 770 | 235 | 250 | 380 | ||||||||||||||
| Nb | 70·0 | 86·0 | 73·0 | 94·0 | 89·0 | 95·0 | 103·0 | 120·0 | 134·0 | 37·0 | 36·0 | 66·0 | ||||||||||||
| La | 56·0 | 71·5 | 57·0 | 76·7 | 65·0 | 69·0 | 85·0 | 73·0 | 106·0 | 25·0 | 27·0 | 62·0 | ||||||||||||
| Ce | 118·0 | 146·8 | 117·0 | 152·1 | 124·0 | 132·0 | 148·0 | 133·0 | 210·0 | 58·0 | 63·0 | 130·0 | ||||||||||||
| Nd | 60·5 | 68·7 | 58·0 | 66·9 | 50·0 | 55·0 | 62·0 | 45·0 | 86·0 | 34·0 | 41·0 | 69·0 | ||||||||||||
| Sm | 11·50 | 13·05 | 11·20 | 12·91 | 8·90 | 9·50 | 10·00 | 7·10 | 14·70 | 7·50 | 10·80 | 13·20 | ||||||||||||
| Eu | 3·30 | 3·84 | 3·20 | 3·80 | 2·63 | 2·75 | 2·96 | 2·00 | 3·17 | 2·50 | 2·55 | 4·02 | ||||||||||||
| Gd | 9·90 | 10·99 | 9·75 | 10·93 | 7·00 | 7·60 | 7·80 | 5·60 | 10·80 | 6·85 | 7·70 | 11·10 | ||||||||||||
| Dy | 7·30 | 8·11 | 7·10 | 7·96 | 5·50 | 5·70 | 5·90 | 4·50 | 8·70 | 4·80 | 5·20 | 7·80 | ||||||||||||
| Er | 3·20 | 3·55 | 3·20 | 3·58 | 2·35 | 3·00 | 2·90 | 2·40 | 4·00 | 1·90 | 2·00 | 3·10 | ||||||||||||
| Yb | 2·38 | 2·62 | 2·59 | 2·87 | 2·10 | 2·39 | 2·23 | 2·23 | 3·40 | 1·40 | 1·50 | 2·24 | ||||||||||||
| Th | 6·15 | 7·99 | 5·41 | 7·45 | 9·60 | 8·80 | 9·90 | 14·00 | 19·40 | 2·55 | 2·70 | 7·60 | ||||||||||||
| 87Sr/86Sr | 0·703965 ± 9 | 0·703937 ± 8 | 0·703958 ± 9 | 0·704433 ± 10 | 0·704434 ± 6 | 0·704367 ± 10 | ||||||||||||||||||
| 143Nd/144Nd | 0·512880 ± 2 | 0·512886 ± 5 | 0·512887 ± 1 | 0·512819 ± 10 | 0·512823 ± 3 | 0·512823 ± 9 | ||||||||||||||||||
| Island: . | Tahiti . | Tahiti . | Tahiti . | Tahiti . | Tahiti . | Tahiti . | Tahiti . | Tahiti . | Tahiti . | Raiatea . | Raiatea . | Raiatea . | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample: . | THG-11A . | THG-11A* . | THG-3A . | THG-3A* . | THG-4 . | THG-6A . | THG-3B . | THG-19 . | THG-1Db . | RIG-3B . | RI-28 . | RIG-1B . | ||||||||||||
| Type: . | Ess . | Ess . | Ess . | Ess . | Ne-Msye . | Ne-Msye . | Ne-Sye . | Ne-Sye . | Ne-Sye . | Gab . | Gab . | The . | ||||||||||||
| Group: . | 1 . | 1 . | 1 . | 1 . | 1 . | 1 . | 1 . | 1 . | 1 . | . | . | . | ||||||||||||
| Reference: . | a . | . | a . | . | a . | a . | a . | a . | a . | a . | a, e . | a . | ||||||||||||
| SiO2 | 42·40 | 44·43 | 42·30 | 44·74 | 49·00 | 48·00 | 50·70 | 52·30 | 54·00 | 44·30 | 44·50 | 47·20 | ||||||||||||
| TiO2 | 3·59 | 2·79 | 3·65 | 2·81 | 2·01 | 2·29 | 1·55 | 1·15 | 1·42 | 6·30 | 6·05 | 3·04 | ||||||||||||
| Al2O3 | 15·35 | 18·52 | 15·00 | 18·99 | 19·05 | 18·60 | 20·45 | 20·30 | 18·60 | 15·00 | 14·43 | 18·50 | ||||||||||||
| Fe2O3t | 12·70 | 9·74 | 13·10 | 9·81 | 7·42 | 8·85 | 5·42 | 4·48 | 6·90 | 12·40 | 12·77 | 10·45 | ||||||||||||
| MnO | 0·20 | 0·11 | 0·22 | 0·12 | 0·16 | 0·19 | 0·13 | 0·16 | 0·12 | 0·15 | 0·15 | 0·15 | ||||||||||||
| MgO | 5·88 | 4·15 | 6·25 | 4·19 | 2·78 | 3·05 | 1·22 | 0·89 | 1·21 | 12·00 | 11·50 | 8·10 | ||||||||||||
| CaO | 10·70 | 8·54 | 11·30 | 8·49 | 6·13 | 6·45 | 4·95 | 3·70 | 3·12 | 12·00 | 11·50 | 8·10 | ||||||||||||
| Na2O | 4·45 | 5·81 | 3·90 | 5·46 | 6·40 | 6·20 | 5·95 | 7·00 | 6·52 | 2·80 | 2·82 | 5·00 | ||||||||||||
| K2O | 2·63 | 3·52 | 1·84 | 2·67 | 3·88 | 3·95 | 4·88 | 6·10 | 6·70 | 1·05 | 1·18 | 3·10 | ||||||||||||
| P2O5 | 0·85 | 1·14 | 0·64 | 0·93 | 0·56 | 0·53 | 0·28 | 0·21 | 0·34 | 0·38 | 0·40 | 1·00 | ||||||||||||
| LOI | 1·08 | 1·08 | 1·20 | 1·20 | 2·37 | 1·80 | 4·34 | 3·63 | 1·09 | 0·25 | 0·32 | 0·44 | ||||||||||||
| Total | 99·83 | 99·83 | 99·40 | 99·40 | 99·76 | 99·91 | 99·87 | 99·92 | 100·02 | 100·23 | 99·86 | 100·03 | ||||||||||||
| Q | ||||||||||||||||||||||||
| Hy | ||||||||||||||||||||||||
| Ne + Lc | 18·8 | 23·1 | 14·1 | 17·6 | 18·6 | 19·8 | 14·7 | 21·5 | 17·3 | 3·2 | 2·8 | 12·9 | ||||||||||||
| Rb | 75·5 | 99·3 | 44·0 | 61·5 | 121·0 | 123·0 | 152·0 | 205·0 | 155·0 | 20·5 | 26·0 | 73·0 | ||||||||||||
| Sr | 905 | 1157 | 1035 | 1391 | 1020 | 1010 | 1465 | 890 | 495 | 870 | 800 | 1230 | ||||||||||||
| Ba | 740 | 979 | 720 | 1013 | 1140 | 1050 | 1350 | 1270 | 1050 | 285 | 302 | 740 | ||||||||||||
| Sc | 17·8 | 21·0 | 5·8 | 6·8 | 0·8 | 0·4 | 2·5 | 25·0 | 25·5 | 6·0 | ||||||||||||||
| V | 333 | 320 | 145 | 158 | 82 | 55 | 28 | 425 | 430 | 140 | ||||||||||||||
| Cr | 63 | 102 | 30 | 26 | 1 | 3 | 2 | 54 | 65 | 7 | ||||||||||||||
| Co | 39 | 40 | 17 | 21 | 6 | 4 | 9 | 40 | 42 | 25 | ||||||||||||||
| Ni | 55 | 61 | 17 | 22 | 2 | 2 | 4 | 95 | 97 | 25 | ||||||||||||||
| Y | 34·8 | 38·6 | 34·0 | 38·1 | 28·5 | 30·0 | 32·0 | 26·0 | 46·0 | 22·5 | 25·0 | 36·5 | ||||||||||||
| Zr | 350 | 315 | 280 | 270 | 335 | 495 | 770 | 235 | 250 | 380 | ||||||||||||||
| Nb | 70·0 | 86·0 | 73·0 | 94·0 | 89·0 | 95·0 | 103·0 | 120·0 | 134·0 | 37·0 | 36·0 | 66·0 | ||||||||||||
| La | 56·0 | 71·5 | 57·0 | 76·7 | 65·0 | 69·0 | 85·0 | 73·0 | 106·0 | 25·0 | 27·0 | 62·0 | ||||||||||||
| Ce | 118·0 | 146·8 | 117·0 | 152·1 | 124·0 | 132·0 | 148·0 | 133·0 | 210·0 | 58·0 | 63·0 | 130·0 | ||||||||||||
| Nd | 60·5 | 68·7 | 58·0 | 66·9 | 50·0 | 55·0 | 62·0 | 45·0 | 86·0 | 34·0 | 41·0 | 69·0 | ||||||||||||
| Sm | 11·50 | 13·05 | 11·20 | 12·91 | 8·90 | 9·50 | 10·00 | 7·10 | 14·70 | 7·50 | 10·80 | 13·20 | ||||||||||||
| Eu | 3·30 | 3·84 | 3·20 | 3·80 | 2·63 | 2·75 | 2·96 | 2·00 | 3·17 | 2·50 | 2·55 | 4·02 | ||||||||||||
| Gd | 9·90 | 10·99 | 9·75 | 10·93 | 7·00 | 7·60 | 7·80 | 5·60 | 10·80 | 6·85 | 7·70 | 11·10 | ||||||||||||
| Dy | 7·30 | 8·11 | 7·10 | 7·96 | 5·50 | 5·70 | 5·90 | 4·50 | 8·70 | 4·80 | 5·20 | 7·80 | ||||||||||||
| Er | 3·20 | 3·55 | 3·20 | 3·58 | 2·35 | 3·00 | 2·90 | 2·40 | 4·00 | 1·90 | 2·00 | 3·10 | ||||||||||||
| Yb | 2·38 | 2·62 | 2·59 | 2·87 | 2·10 | 2·39 | 2·23 | 2·23 | 3·40 | 1·40 | 1·50 | 2·24 | ||||||||||||
| Th | 6·15 | 7·99 | 5·41 | 7·45 | 9·60 | 8·80 | 9·90 | 14·00 | 19·40 | 2·55 | 2·70 | 7·60 | ||||||||||||
| 87Sr/86Sr | 0·703965 ± 9 | 0·703937 ± 8 | 0·703958 ± 9 | 0·704433 ± 10 | 0·704434 ± 6 | 0·704367 ± 10 | ||||||||||||||||||
| 143Nd/144Nd | 0·512880 ± 2 | 0·512886 ± 5 | 0·512887 ± 1 | 0·512819 ± 10 | 0·512823 ± 3 | 0·512823 ± 9 | ||||||||||||||||||
| Island: . | Raiatea . | Raiatea . | Raiatea . | Raiatea . | Raiatea . | Raiatea . | Raiatea . | Raiatea . | Raiatea . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample: . | RIG-1E . | RIG-2A . | RIG-2C . | RIG-2D . | RIG-4A . | RIG-4B . | RIG-4C . | RI-85 . | RI-86 . | |||||||||
| Type: . | The . | The . | The . | The . | The . | The . | The . | The . | The . | |||||||||
| Group: . | . | . | . | . | . | . | . | . | . | |||||||||
| Reference: . | a . | a . | a . | a . | a . | a . | a . | a, e . | a, e . | |||||||||
| SiO2 | 44·60 | 47·60 | 47·00 | 46·80 | 46·40 | 47·30 | 42·00 | 45·80 | 46·60 | |||||||||
| TiO2 | 4·24 | 2·94 | 3·18 | 3·30 | 4·19 | 3·30 | 5·34 | 3·62 | 3·34 | |||||||||
| Al2O3 | 17·45 | 18·60 | 17·80 | 17·90 | 16·60 | 16·80 | 13·70 | 17·08 | 18·35 | |||||||||
| Fe2O3t | 12·30 | 10·00 | 10·20 | 10·85 | 11·90 | 11·25 | 15·30 | 11·18 | 10·75 | |||||||||
| MnO | 0·15 | 0·14 | 0·15 | 0·15 | 0·15 | 0·16 | 0·19 | 0·16 | 0·14 | |||||||||
| MgO | 9·90 | 8·50 | 8·70 | 8·60 | 3·58 | 4·08 | 6·40 | 3·33 | 3·17 | |||||||||
| CaO | 9·90 | 8·50 | 8·70 | 8·60 | 9·55 | 8·10 | 10·00 | 10·00 | 9·72 | |||||||||
| Na2O | 4·30 | 5·15 | 5·00 | 4·58 | 4·50 | 4·80 | 3·70 | 4·09 | 4·25 | |||||||||
| K2O | 1·95 | 2·60 | 2·75 | 3·20 | 2·20 | 2·48 | 1·58 | 2·35 | 2·23 | |||||||||
| P2O5 | 1·05 | 1·00 | 1·15 | 0·99 | 0·80 | 0·83 | 0·80 | 1·12 | 0·82 | |||||||||
| LOI | 0·21 | 0·52 | 0·84 | 0·44 | 0·04 | 0·76 | 0·70 | 1·14 | 0·91 | |||||||||
| Total | 100·03 | 99·93 | 99·92 | 99·96 | 99·91 | 99·86 | 99·71 | 99·87 | 100·28 | |||||||||
| Q | ||||||||||||||||||
| Hy | ||||||||||||||||||
| Ne + Lc | 10·6 | 11·9 | 12·2 | 12·0 | 10·0 | 9·9 | 10·6 | 8·8 | 8·8 | |||||||||
| Rb | 37·5 | 53·0 | 60·0 | 85·5 | 47·0 | 65·0 | 31·5 | 52·0 | 50·0 | |||||||||
| Sr | 1160 | 1250 | 1220 | 1170 | 970 | 1100 | 1020 | 1165 | 1190 | |||||||||
| Ba | 560 | 730 | 715 | 690 | 530 | 700 | 430 | 642 | 585 | |||||||||
| Sc | 10·8 | 6·0 | 7·5 | 7·3 | 11·2 | 9·5 | 13·0 | 10·0 | 8·8 | |||||||||
| V | 255 | 135 | 138 | 155 | 285 | 225 | 365 | 154 | 175 | |||||||||
| Cr | 10 | 6 | 5 | 30 | 2 | 38 | 95 | 7 | 10 | |||||||||
| Co | 34 | 22 | 22 | 24 | 27 | 30 | 49 | 25 | 26 | |||||||||
| Ni | 38 | 20 | 20 | 23 | 38 | 50 | 127 | 18 | 23 | |||||||||
| Y | 33·0 | 36·5 | 37·0 | 38·0 | 39·5 | 34·0 | 31·0 | 40·0 | 34·0 | |||||||||
| Zr | 335 | 370 | 376 | 370 | 386 | 420 | 365 | 363 | 340 | |||||||||
| Nb | 55·0 | 64·0 | 64·0 | 66·0 | 60·0 | 79·0 | 66·0 | 67·0 | 58·0 | |||||||||
| La | 52·0 | 62·5 | 62·0 | 60·0 | 54·0 | 65·0 | 52·0 | 61·0 | 52·5 | |||||||||
| Ce | 115·0 | 132·0 | 133·0 | 130·0 | 119·0 | 136·0 | 111·0 | 133·0 | 110·0 | |||||||||
| Nd | 62·0 | 67·5 | 69·0 | 67·0 | 66·0 | 65·0 | 62·5 | 76·0 | 63·0 | |||||||||
| Sm | 12·4 | 13·10 | 13·80 | 13·20 | 13·60 | 12·30 | 12·20 | 17·10 | 13·10 | |||||||||
| Eu | 3·72 | 3·93 | 4·12 | 3·92 | 4·00 | 3·70 | 3·67 | 4·25 | 3·50 | |||||||||
| Gd | 10·75 | 11·10 | 11·90 | 11·20 | 11·50 | 10·30 | 10·10 | 12·80 | 10·60 | |||||||||
| Dy | 7·40 | 7·60 | 7·80 | 7·80 | 8·20 | 7·05 | 7·10 | 8·30 | 6·90 | |||||||||
| Er | 2·80 | 3·00 | 3·10 | 3·00 | 3·20 | 2·90 | 2·80 | 3·50 | 2·80 | |||||||||
| Yb | 2·03 | 2·25 | 2·27 | 2·29 | 2·44 | 2·18 | 1·76 | 2·35 | 2·10 | |||||||||
| Th | 6·00 | 7·95 | 7·65 | 7·80 | 6·10 | 8·70 | 6·50 | 6·80 | 6·70 | |||||||||
| 87Sr/86Sr | 0·704085 ± 5 | |||||||||||||||||
| 143Nd/144Nd | 0·512853 ± 2 | |||||||||||||||||
| Island: . | Raiatea . | Raiatea . | Raiatea . | Raiatea . | Raiatea . | Raiatea . | Raiatea . | Raiatea . | Raiatea . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample: . | RIG-1E . | RIG-2A . | RIG-2C . | RIG-2D . | RIG-4A . | RIG-4B . | RIG-4C . | RI-85 . | RI-86 . | |||||||||
| Type: . | The . | The . | The . | The . | The . | The . | The . | The . | The . | |||||||||
| Group: . | . | . | . | . | . | . | . | . | . | |||||||||
| Reference: . | a . | a . | a . | a . | a . | a . | a . | a, e . | a, e . | |||||||||
| SiO2 | 44·60 | 47·60 | 47·00 | 46·80 | 46·40 | 47·30 | 42·00 | 45·80 | 46·60 | |||||||||
| TiO2 | 4·24 | 2·94 | 3·18 | 3·30 | 4·19 | 3·30 | 5·34 | 3·62 | 3·34 | |||||||||
| Al2O3 | 17·45 | 18·60 | 17·80 | 17·90 | 16·60 | 16·80 | 13·70 | 17·08 | 18·35 | |||||||||
| Fe2O3t | 12·30 | 10·00 | 10·20 | 10·85 | 11·90 | 11·25 | 15·30 | 11·18 | 10·75 | |||||||||
| MnO | 0·15 | 0·14 | 0·15 | 0·15 | 0·15 | 0·16 | 0·19 | 0·16 | 0·14 | |||||||||
| MgO | 9·90 | 8·50 | 8·70 | 8·60 | 3·58 | 4·08 | 6·40 | 3·33 | 3·17 | |||||||||
| CaO | 9·90 | 8·50 | 8·70 | 8·60 | 9·55 | 8·10 | 10·00 | 10·00 | 9·72 | |||||||||
| Na2O | 4·30 | 5·15 | 5·00 | 4·58 | 4·50 | 4·80 | 3·70 | 4·09 | 4·25 | |||||||||
| K2O | 1·95 | 2·60 | 2·75 | 3·20 | 2·20 | 2·48 | 1·58 | 2·35 | 2·23 | |||||||||
| P2O5 | 1·05 | 1·00 | 1·15 | 0·99 | 0·80 | 0·83 | 0·80 | 1·12 | 0·82 | |||||||||
| LOI | 0·21 | 0·52 | 0·84 | 0·44 | 0·04 | 0·76 | 0·70 | 1·14 | 0·91 | |||||||||
| Total | 100·03 | 99·93 | 99·92 | 99·96 | 99·91 | 99·86 | 99·71 | 99·87 | 100·28 | |||||||||
| Q | ||||||||||||||||||
| Hy | ||||||||||||||||||
| Ne + Lc | 10·6 | 11·9 | 12·2 | 12·0 | 10·0 | 9·9 | 10·6 | 8·8 | 8·8 | |||||||||
| Rb | 37·5 | 53·0 | 60·0 | 85·5 | 47·0 | 65·0 | 31·5 | 52·0 | 50·0 | |||||||||
| Sr | 1160 | 1250 | 1220 | 1170 | 970 | 1100 | 1020 | 1165 | 1190 | |||||||||
| Ba | 560 | 730 | 715 | 690 | 530 | 700 | 430 | 642 | 585 | |||||||||
| Sc | 10·8 | 6·0 | 7·5 | 7·3 | 11·2 | 9·5 | 13·0 | 10·0 | 8·8 | |||||||||
| V | 255 | 135 | 138 | 155 | 285 | 225 | 365 | 154 | 175 | |||||||||
| Cr | 10 | 6 | 5 | 30 | 2 | 38 | 95 | 7 | 10 | |||||||||
| Co | 34 | 22 | 22 | 24 | 27 | 30 | 49 | 25 | 26 | |||||||||
| Ni | 38 | 20 | 20 | 23 | 38 | 50 | 127 | 18 | 23 | |||||||||
| Y | 33·0 | 36·5 | 37·0 | 38·0 | 39·5 | 34·0 | 31·0 | 40·0 | 34·0 | |||||||||
| Zr | 335 | 370 | 376 | 370 | 386 | 420 | 365 | 363 | 340 | |||||||||
| Nb | 55·0 | 64·0 | 64·0 | 66·0 | 60·0 | 79·0 | 66·0 | 67·0 | 58·0 | |||||||||
| La | 52·0 | 62·5 | 62·0 | 60·0 | 54·0 | 65·0 | 52·0 | 61·0 | 52·5 | |||||||||
| Ce | 115·0 | 132·0 | 133·0 | 130·0 | 119·0 | 136·0 | 111·0 | 133·0 | 110·0 | |||||||||
| Nd | 62·0 | 67·5 | 69·0 | 67·0 | 66·0 | 65·0 | 62·5 | 76·0 | 63·0 | |||||||||
| Sm | 12·4 | 13·10 | 13·80 | 13·20 | 13·60 | 12·30 | 12·20 | 17·10 | 13·10 | |||||||||
| Eu | 3·72 | 3·93 | 4·12 | 3·92 | 4·00 | 3·70 | 3·67 | 4·25 | 3·50 | |||||||||
| Gd | 10·75 | 11·10 | 11·90 | 11·20 | 11·50 | 10·30 | 10·10 | 12·80 | 10·60 | |||||||||
| Dy | 7·40 | 7·60 | 7·80 | 7·80 | 8·20 | 7·05 | 7·10 | 8·30 | 6·90 | |||||||||
| Er | 2·80 | 3·00 | 3·10 | 3·00 | 3·20 | 2·90 | 2·80 | 3·50 | 2·80 | |||||||||
| Yb | 2·03 | 2·25 | 2·27 | 2·29 | 2·44 | 2·18 | 1·76 | 2·35 | 2·10 | |||||||||
| Th | 6·00 | 7·95 | 7·65 | 7·80 | 6·10 | 8·70 | 6·50 | 6·80 | 6·70 | |||||||||
| 87Sr/86Sr | 0·704085 ± 5 | |||||||||||||||||
| 143Nd/144Nd | 0·512853 ± 2 | |||||||||||||||||
Accumulation-free chemical compositions estimated by using the MONA program (see text for explanations).
Abbreviations as in Table 1. Q, Hy, Ne, and Lc are CIPW normative mineral proportions calculated with Fe2O3/FeO varying with the petrographic type (Middlemost, 1989). Group 1 corresponds to the strongly Si-undersaturated suite, Group 2 to the mildly Si-undersaturated suite, and Group 3 includes strongly Si-undersaturated rocks with low 87Sr/86Sr and high 143Nd/144Nd. References: a, this work; b, Clément et al. (2002); c, Bardintzeff et al. (1988); d, Nitecki-Novotni (1975); e, Blais et al. (1997) (major elements).
Major and trace elements, and Nd–Sr isotopes from Ahititera (Tahiti Nui) and Faaroa (Raiatea) samples
| Island: . | Tahiti . | Tahiti . | Tahiti . | Tahiti . | Tahiti . | Tahiti . | Tahiti . | Tahiti . | Tahiti . | Tahiti . | Tahiti . | Tahiti . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample: . | THG-10B . | THG-5C . | THG-10C . | THG-10E . | THG-1B . | THG-1 Da . | THG-7B . | TP6 . | 37H . | THG-9B . | THG-2D . | THG-14 . |
| Type: . | Ol-Cpxite . | Ol-Cpxite . | Gab . | Gab . | Gab . | Gab . | Gab . | Monz . | Monz . | Alk-Sye . | Cpx-Hblite . | Cpx-Hblite . |
| Group: . | 2 . | 2 . | 2 . | 2 . | 2 . | 2 . | 2 . | 2 . | 2 . | 2 . | 1 . | 1 . |
| Reference: . | a . | a . | b . | a . | a . | a . | a . | c . | d . | a . | a . | a . |
| SiO2 | 41·40 | 43·80 | 45·30 | 40·40 | 44·00 | 42·80 | 43·00 | 46·30 | 49·23 | 61·00 | 36·00 | 38·60 |
| TiO2 | 1·90 | 2·52 | 3·36 | 6·60 | 5·35 | 4·10 | 4·52 | 4·27 | 3·33 | 0·65 | 5·45 | 4·59 |
| Al2O3 | 6·08 | 9·65 | 13·90 | 13·70 | 15·00 | 16·10 | 13·72 | 17·97 | 17·24 | 18·40 | 14·35 | 11·70 |
| Fe2O3t | 14·70 | 12·45 | 14·00 | 13·45 | 13·70 | 14·20 | 15·60 | 11·95 | 10·30 | 4·90 | 15·60 | 15·60 |
| MnO | 0·20 | 0·19 | 0·15 | 0·16 | 0·17 | 0·17 | 0·18 | 0·19 | 0·16 | 0·03 | 0·21 | 0·22 |
| MgO | 20·30 | 13·20 | 6·47 | 8·04 | 6·02 | 5·60 | 6·55 | 2·77 | 4·26 | 0·47 | 7·95 | 9·50 |
| CaO | 10·50 | 14·65 | 11·35 | 14·55 | 10·50 | 11·90 | 12·25 | 7·62 | 7·13 | 0·37 | 14·40 | 13·85 |
| Na2O | 0·52 | 1·06 | 3·12 | 1·76 | 3·00 | 2·70 | 2·32 | 3·03 | 4·41 | 7·30 | 2·43 | 2·35 |
| K2O | 0·53 | 0·41 | 1·13 | 0·56 | 1·78 | 1·08 | 0·90 | 2·41 | 1·88 | 4·90 | 1·22 | 1·45 |
| P2O5 | 0·20 | 0·15 | 0·46 | 0·17 | 0·40 | 0·32 | 0·42 | 0·96 | n.d. | 0·13 | 1·90 | 0·83 |
| LOI | 3·25 | 1·68 | 0·79 | 0·42 | 0·44 | 0·15 | 0·45 | 1·81 | 1·99 | 1·51 | 0·35 | 0·91 |
| Total | 99·58 | 99·76 | 100·03 | 99·81 | 100·36 | 99·12 | 99·91 | 99·28 | 99·93 | 99·66 | 99·86 | 99·60 |
| Q | 0·5 | |||||||||||
| Hy | 9·8 | 1·7 | ||||||||||
| Ne + Lc | 0·3 | 2·3 | 5·2 | 6·5 | 6·2 | 6·4 | 3·8 | 0·0 | 0·0 | 1·4 | 16·8 | 17·5 |
| Rb | 12·0 | 8·2 | 26·0 | 18·0 | 40·5 | 22·5 | 19·0 | 75 | 61·7 | 104·0 | 20·5 | 40·0 |
| Sr | 166 | 424 | 700 | 750 | 795 | 910 | 810 | 1134 | 858 | 270 | 1390 | 720 |
| Ba | 75 | 185 | 360 | 142 | 360 | 33 | 320 | 692 | 868 | 1130 | 650 | 540 |
| Sc | 34·0 | 45·0 | 28·0 | 35·5 | 24·0 | 21·0 | 26·0 | n.d. | 722·0 | 0·6 | 14·0 | 26·0 |
| V | 240 | 310 | 420 | 425 | 419 | 480 | 560 | n.d. | 254 | 8 | 440 | 460 |
| Cr | 1260 | 900 | 80 | 175 | 78 | 46 | 85 | 12 | 127 | 4 | 41 | 209 |
| Co | 86 | 59 | 51 | 47 | 46 | 51 | 52 | 44 | 18 | 2 | 41 | 54 |
| Ni | 660 | 300 | 165 | 130 | 92 | 110 | 115 | 54 | 7 | 4 | 50 | 145 |
| Y | 14·0 | 19·3 | 28·0 | 20·8 | 24·0 | 22·0 | 27·5 | n.d. | n.d. | 21·5 | 49·0 | 38·5 |
| Zr | 68 | 137 | 175 | 223 | 270 | 215 | 258 | n.d. | n.d. | 420 | 235 | 300 |
| Nb | 11·6 | 11·0 | 37·0 | 30·5 | 47·0 | 34·0 | 32·0 | n.d. | n.d. | 83·0 | 55·0 | 57·0 |
| La | 11·0 | 12·6 | 31·0 | 18·0 | 33·5 | 25·0 | 29·5 | n.d. | 85·3 | 78·0 | 60·0 | 46·0 |
| Ce | 27·0 | 34·0 | 70·0 | 42·5 | 74·0 | 57·0 | 70·0 | n.d. | 130·0 | 148·0 | 142·0 | 106·0 |
| Nd | 18·0 | 24·0 | 42·0 | 29·5 | 42·0 | 34·0 | 44·0 | n.d. | n.d. | 58·0 | 92·0 | 62·0 |
| Sm | 4·60 | 6·05 | 8·90 | 6·90 | 8·40 | 7·15 | 9·05 | n.d. | 10·70 | 9·10 | 17·60 | 12·90 |
| Eu | 1·30 | 1·95 | 2·62 | 2·07 | 2·48 | 2·45 | 2·89 | n.d. | 5·00 | 2·54 | 5·37 | 3·73 |
| Gd | 3·75 | 5·80 | 7·90 | 6·35 | 7·50 | 6·20 | 8·00 | n.d. | n.d. | 6·60 | 16·00 | 11·00 |
| Dy | 3·10 | 4·30 | 5·90 | 4·50 | 5·20 | 4·60 | 6·00 | n.d. | 7·10 | 3·90 | 10·40 | 8·00 |
| Er | 1·40 | 1·70 | 2·60 | 1·80 | 2·10 | 1·90 | 2·50 | n.d. | n.d. | 1·80 | 4·10 | 3·40 |
| Yb | 1·02 | 1·20 | 1·90 | 1·34 | 1·58 | 1·25 | 1·71 | n.d. | 1·12 | 1·75 | 2·72 | 2·36 |
| Th | 0·80 | 0·85 | 3·30 | 2·30 | 4·30 | 2·90 | 3·05 | n.d. | 7·44 | 11·20 | 2·80 | 3·05 |
| 87Sr/86Sr | 0·704566 ± 10 | 0·704659 ± 9 | 0·704532 ± 9 | 0·703964 ± 10 | ||||||||
| 143Nd/144Nd | 0·512774 ± 1 | 0·512769 ± 7 | 0·512782 ± 3 | 0·512893 ± 2 |
| Island: . | Tahiti . | Tahiti . | Tahiti . | Tahiti . | Tahiti . | Tahiti . | Tahiti . | Tahiti . | Tahiti . | Tahiti . | Tahiti . | Tahiti . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample: . | THG-10B . | THG-5C . | THG-10C . | THG-10E . | THG-1B . | THG-1 Da . | THG-7B . | TP6 . | 37H . | THG-9B . | THG-2D . | THG-14 . |
| Type: . | Ol-Cpxite . | Ol-Cpxite . | Gab . | Gab . | Gab . | Gab . | Gab . | Monz . | Monz . | Alk-Sye . | Cpx-Hblite . | Cpx-Hblite . |
| Group: . | 2 . | 2 . | 2 . | 2 . | 2 . | 2 . | 2 . | 2 . | 2 . | 2 . | 1 . | 1 . |
| Reference: . | a . | a . | b . | a . | a . | a . | a . | c . | d . | a . | a . | a . |
| SiO2 | 41·40 | 43·80 | 45·30 | 40·40 | 44·00 | 42·80 | 43·00 | 46·30 | 49·23 | 61·00 | 36·00 | 38·60 |
| TiO2 | 1·90 | 2·52 | 3·36 | 6·60 | 5·35 | 4·10 | 4·52 | 4·27 | 3·33 | 0·65 | 5·45 | 4·59 |
| Al2O3 | 6·08 | 9·65 | 13·90 | 13·70 | 15·00 | 16·10 | 13·72 | 17·97 | 17·24 | 18·40 | 14·35 | 11·70 |
| Fe2O3t | 14·70 | 12·45 | 14·00 | 13·45 | 13·70 | 14·20 | 15·60 | 11·95 | 10·30 | 4·90 | 15·60 | 15·60 |
| MnO | 0·20 | 0·19 | 0·15 | 0·16 | 0·17 | 0·17 | 0·18 | 0·19 | 0·16 | 0·03 | 0·21 | 0·22 |
| MgO | 20·30 | 13·20 | 6·47 | 8·04 | 6·02 | 5·60 | 6·55 | 2·77 | 4·26 | 0·47 | 7·95 | 9·50 |
| CaO | 10·50 | 14·65 | 11·35 | 14·55 | 10·50 | 11·90 | 12·25 | 7·62 | 7·13 | 0·37 | 14·40 | 13·85 |
| Na2O | 0·52 | 1·06 | 3·12 | 1·76 | 3·00 | 2·70 | 2·32 | 3·03 | 4·41 | 7·30 | 2·43 | 2·35 |
| K2O | 0·53 | 0·41 | 1·13 | 0·56 | 1·78 | 1·08 | 0·90 | 2·41 | 1·88 | 4·90 | 1·22 | 1·45 |
| P2O5 | 0·20 | 0·15 | 0·46 | 0·17 | 0·40 | 0·32 | 0·42 | 0·96 | n.d. | 0·13 | 1·90 | 0·83 |
| LOI | 3·25 | 1·68 | 0·79 | 0·42 | 0·44 | 0·15 | 0·45 | 1·81 | 1·99 | 1·51 | 0·35 | 0·91 |
| Total | 99·58 | 99·76 | 100·03 | 99·81 | 100·36 | 99·12 | 99·91 | 99·28 | 99·93 | 99·66 | 99·86 | 99·60 |
| Q | 0·5 | |||||||||||
| Hy | 9·8 | 1·7 | ||||||||||
| Ne + Lc | 0·3 | 2·3 | 5·2 | 6·5 | 6·2 | 6·4 | 3·8 | 0·0 | 0·0 | 1·4 | 16·8 | 17·5 |
| Rb | 12·0 | 8·2 | 26·0 | 18·0 | 40·5 | 22·5 | 19·0 | 75 | 61·7 | 104·0 | 20·5 | 40·0 |
| Sr | 166 | 424 | 700 | 750 | 795 | 910 | 810 | 1134 | 858 | 270 | 1390 | 720 |
| Ba | 75 | 185 | 360 | 142 | 360 | 33 | 320 | 692 | 868 | 1130 | 650 | 540 |
| Sc | 34·0 | 45·0 | 28·0 | 35·5 | 24·0 | 21·0 | 26·0 | n.d. | 722·0 | 0·6 | 14·0 | 26·0 |
| V | 240 | 310 | 420 | 425 | 419 | 480 | 560 | n.d. | 254 | 8 | 440 | 460 |
| Cr | 1260 | 900 | 80 | 175 | 78 | 46 | 85 | 12 | 127 | 4 | 41 | 209 |
| Co | 86 | 59 | 51 | 47 | 46 | 51 | 52 | 44 | 18 | 2 | 41 | 54 |
| Ni | 660 | 300 | 165 | 130 | 92 | 110 | 115 | 54 | 7 | 4 | 50 | 145 |
| Y | 14·0 | 19·3 | 28·0 | 20·8 | 24·0 | 22·0 | 27·5 | n.d. | n.d. | 21·5 | 49·0 | 38·5 |
| Zr | 68 | 137 | 175 | 223 | 270 | 215 | 258 | n.d. | n.d. | 420 | 235 | 300 |
| Nb | 11·6 | 11·0 | 37·0 | 30·5 | 47·0 | 34·0 | 32·0 | n.d. | n.d. | 83·0 | 55·0 | 57·0 |
| La | 11·0 | 12·6 | 31·0 | 18·0 | 33·5 | 25·0 | 29·5 | n.d. | 85·3 | 78·0 | 60·0 | 46·0 |
| Ce | 27·0 | 34·0 | 70·0 | 42·5 | 74·0 | 57·0 | 70·0 | n.d. | 130·0 | 148·0 | 142·0 | 106·0 |
| Nd | 18·0 | 24·0 | 42·0 | 29·5 | 42·0 | 34·0 | 44·0 | n.d. | n.d. | 58·0 | 92·0 | 62·0 |
| Sm | 4·60 | 6·05 | 8·90 | 6·90 | 8·40 | 7·15 | 9·05 | n.d. | 10·70 | 9·10 | 17·60 | 12·90 |
| Eu | 1·30 | 1·95 | 2·62 | 2·07 | 2·48 | 2·45 | 2·89 | n.d. | 5·00 | 2·54 | 5·37 | 3·73 |
| Gd | 3·75 | 5·80 | 7·90 | 6·35 | 7·50 | 6·20 | 8·00 | n.d. | n.d. | 6·60 | 16·00 | 11·00 |
| Dy | 3·10 | 4·30 | 5·90 | 4·50 | 5·20 | 4·60 | 6·00 | n.d. | 7·10 | 3·90 | 10·40 | 8·00 |
| Er | 1·40 | 1·70 | 2·60 | 1·80 | 2·10 | 1·90 | 2·50 | n.d. | n.d. | 1·80 | 4·10 | 3·40 |
| Yb | 1·02 | 1·20 | 1·90 | 1·34 | 1·58 | 1·25 | 1·71 | n.d. | 1·12 | 1·75 | 2·72 | 2·36 |
| Th | 0·80 | 0·85 | 3·30 | 2·30 | 4·30 | 2·90 | 3·05 | n.d. | 7·44 | 11·20 | 2·80 | 3·05 |
| 87Sr/86Sr | 0·704566 ± 10 | 0·704659 ± 9 | 0·704532 ± 9 | 0·703964 ± 10 | ||||||||
| 143Nd/144Nd | 0·512774 ± 1 | 0·512769 ± 7 | 0·512782 ± 3 | 0·512893 ± 2 |
| Island: . | Tahiti . | Tahiti . | Tahiti . | Tahiti . | Tahiti . | Tahiti . | Tahiti . | Tahiti . | Tahiti . | Tahiti . | Tahiti . | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample: . | THG-7A . | THG-13A . | THG-9E2 . | THG-2B . | THG-2A . | THG-2A* . | THG-1A . | THG-1A* . | THG-2C . | THG-9E1 . | THG-1E . | |||||||||||
| Type: . | Cpx-Hblite . | The . | The . | The . | The . | The . | The . | The . | Ess . | Ess . | Ess . | |||||||||||
| Group: . | 1 . | 1 . | 1 . | 3 . | 3 . | 3 . | 3 . | 3 . | 1 . | 1 . | 1 . | |||||||||||
| Reference: . | a . | b . | a . | b . | a . | . | a . | . | a . | b . | a . | |||||||||||
| SiO2 | 37·30 | 44·70 | 43·40 | 46·50 | 45·30 | 46·06 | 45·00 | 46·95 | 44·70 | 45·50 | 44·50 | |||||||||||
| TiO2 | 5·35 | 3·88 | 3·33 | 3·85 | 3·30 | 4·21 | 2·70 | 3·93 | 2·85 | 2·75 | 3·23 | |||||||||||
| Al2O3 | 12·45 | 15·85 | 19·80 | 17·35 | 13·05 | 17·40 | 11·30 | 17·49 | 18·45 | 18·00 | 17·20 | |||||||||||
| Fe2O3t | 17·15 | 12·60 | 12·05 | 11·30 | 12·64 | 11·28 | 13·60 | 11·34 | 9·75 | 9·60 | 10·63 | |||||||||||
| MnO | 0·22 | 0·20 | 0·19 | 0·14 | 0·16 | 0·13 | 0·17 | 0·13 | 0·20 | 0·20 | 0·20 | |||||||||||
| MgO | 8·90 | 4·92 | 3·60 | 3·50 | 9·70 | 3·57 | 13·35 | 3·47 | 3·94 | 3·95 | 4·15 | |||||||||||
| CaO | 12·60 | 9·20 | 9·48 | 9·20 | 9·80 | 9·31 | 8·86 | 9·14 | 8·38 | 8·20 | 8·80 | |||||||||||
| Na2O | 2·28 | 4·30 | 4·47 | 4·40 | 2·93 | 3·90 | 2·53 | 3·91 | 5·30 | 5·25 | 5·80 | |||||||||||
| K2O | 1·46 | 1·85 | 2·02 | 2·40 | 2·10 | 2·91 | 1·39 | 2·25 | 3·46 | 3·70 | 3·50 | |||||||||||
| P2O5 | 0·92 | 0·80 | 0·87 | 0·66 | 0·46 | 0·63 | 0·46 | 0·74 | 0·95 | 0·92 | 0·78 | |||||||||||
| LOI | 1·04 | 1·53 | 0·48 | 0·39 | 0·48 | 0·48 | 0·23 | 0·23 | 2·24 | 1·94 | 1·13 | |||||||||||
| Total | 99·67 | 99·83 | 99·69 | 99·69 | 99·92 | 99·92 | 99·59 | 99·59 | 100·22 | 100·01 | 99·92 | |||||||||||
| Q | ||||||||||||||||||||||
| Hy | ||||||||||||||||||||||
| Ne + Lc | 17·2 | 10·0 | 13·9 | 9·9 | 7·2 | 9·5 | 3·5 | 5·4 | 19·3 | 18·6 | 23·8 | |||||||||||
| Rb | 37·5 | 49·0 | 51·0 | 56·0 | 44·5 | 60·6 | 27·5 | 42·5 | 115·0 | 102·0 | 103·0 | |||||||||||
| Sr | 850 | 1000 | 1590 | 1080 | 750 | 1013 | 560 | 866 | 1470 | 1380 | 1030 | |||||||||||
| Ba | 660 | 730 | 760 | 680 | 515 | 706 | 380 | 595 | 1070 | 1200 | 915 | |||||||||||
| Sc | 23·0 | 17·0 | 6·5 | 11·0 | 22·0 | 22·5 | 2·7 | 4·0 | 10·0 | |||||||||||||
| V | 500 | 310 | 282 | 242 | 270 | 230 | 215 | 195 | 262 | |||||||||||||
| Cr | 170 | 59 | 12 | 10 | 480 | 580 | 1 | 1 | 6 | |||||||||||||
| Co | 55 | 36 | 31 | 29 | 50 | 64 | 17 | 21 | 24 | |||||||||||||
| Ni | 120 | 72 | 21 | 50 | 238 | 407 | 1 | 2 | 17 | |||||||||||||
| Y | 38·0 | 36·0 | 28·0 | 34·0 | 26·5 | 32·5 | 22·0 | 30·3 | 39·0 | 34·0 | 37·5 | |||||||||||
| Zr | 270 | 330 | 255 | 365 | 278 | 215 | 382 | 296 | 405 | |||||||||||||
| Nb | 57·0 | 75·0 | 61·0 | 60·0 | 44·0 | 60·7 | 34·0 | 53·9 | 93·0 | 81·0 | 93·0 | |||||||||||
| La | 42·0 | 57·0 | 54·0 | 64·0 | 45·0 | 61·3 | 34·5 | 53·7 | 78·0 | 70·0 | 69·5 | |||||||||||
| Ce | 100·0 | 120·0 | 109·0 | 128·0 | 96·0 | 128·7 | 74·0 | 113·5 | 165·0 | 142·0 | 140·0 | |||||||||||
| Nd | 66·0 | 60·5 | 53·0 | 65·0 | 50·0 | 62·4 | 41·0 | 57·7 | 79·0 | 67·0 | 70·0 | |||||||||||
| Sm | 13·00 | 11·80 | 9·75 | 11·80 | 9·60 | 11·98 | 8·20 | 11·54 | 13·80 | 11·60 | 12·20 | |||||||||||
| Eu | 3·95 | 3·53 | 3·21 | 3·67 | 2·83 | 3·53 | 2·35 | 3·29 | 3·95 | 3·38 | 3·60 | |||||||||||
| Gd | 11·40 | 10·20 | 8·10 | 10·20 | 8·20 | 10·05 | 7·00 | 9·65 | 11·00 | 9·75 | 10·50 | |||||||||||
| Dy | 8·30 | 7·50 | 5·80 | 7·00 | 5·70 | 6·99 | 4·90 | 6·69 | 8·00 | 6·80 | 7·60 | |||||||||||
| Er | 3·40 | 3·20 | 2·55 | 2·90 | 2·40 | 2·94 | 2·00 | 2·76 | 3·40 | 3·10 | 3·30 | |||||||||||
| Yb | 2·25 | 2·44 | 2·03 | 2·03 | 1·67 | 2·05 | 1·38 | 1·90 | 2·67 | 2·35 | 2·61 | |||||||||||
| Th | 3·15 | 7·45 | 6·10 | 8·80 | 5·90 | 8·11 | 4·45 | 6·99 | 10·40 | 6·80 | 9·15 | |||||||||||
| 87Sr/86Sr | 0·704147 ± 10 | 0·705257 ± 9 | 0·705143 ± 8 | 0·703963 ± 6 | 0·703971 ± 9 | |||||||||||||||||
| 143Nd/144Nd | 0·512884 ± 8 | 0·512718 ± 2 | 0·512729 ± 2 | 0·512883 ± 2 | 0·512892 ± 10 | |||||||||||||||||
| Island: . | Tahiti . | Tahiti . | Tahiti . | Tahiti . | Tahiti . | Tahiti . | Tahiti . | Tahiti . | Tahiti . | Tahiti . | Tahiti . | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample: . | THG-7A . | THG-13A . | THG-9E2 . | THG-2B . | THG-2A . | THG-2A* . | THG-1A . | THG-1A* . | THG-2C . | THG-9E1 . | THG-1E . | |||||||||||
| Type: . | Cpx-Hblite . | The . | The . | The . | The . | The . | The . | The . | Ess . | Ess . | Ess . | |||||||||||
| Group: . | 1 . | 1 . | 1 . | 3 . | 3 . | 3 . | 3 . | 3 . | 1 . | 1 . | 1 . | |||||||||||
| Reference: . | a . | b . | a . | b . | a . | . | a . | . | a . | b . | a . | |||||||||||
| SiO2 | 37·30 | 44·70 | 43·40 | 46·50 | 45·30 | 46·06 | 45·00 | 46·95 | 44·70 | 45·50 | 44·50 | |||||||||||
| TiO2 | 5·35 | 3·88 | 3·33 | 3·85 | 3·30 | 4·21 | 2·70 | 3·93 | 2·85 | 2·75 | 3·23 | |||||||||||
| Al2O3 | 12·45 | 15·85 | 19·80 | 17·35 | 13·05 | 17·40 | 11·30 | 17·49 | 18·45 | 18·00 | 17·20 | |||||||||||
| Fe2O3t | 17·15 | 12·60 | 12·05 | 11·30 | 12·64 | 11·28 | 13·60 | 11·34 | 9·75 | 9·60 | 10·63 | |||||||||||
| MnO | 0·22 | 0·20 | 0·19 | 0·14 | 0·16 | 0·13 | 0·17 | 0·13 | 0·20 | 0·20 | 0·20 | |||||||||||
| MgO | 8·90 | 4·92 | 3·60 | 3·50 | 9·70 | 3·57 | 13·35 | 3·47 | 3·94 | 3·95 | 4·15 | |||||||||||
| CaO | 12·60 | 9·20 | 9·48 | 9·20 | 9·80 | 9·31 | 8·86 | 9·14 | 8·38 | 8·20 | 8·80 | |||||||||||
| Na2O | 2·28 | 4·30 | 4·47 | 4·40 | 2·93 | 3·90 | 2·53 | 3·91 | 5·30 | 5·25 | 5·80 | |||||||||||
| K2O | 1·46 | 1·85 | 2·02 | 2·40 | 2·10 | 2·91 | 1·39 | 2·25 | 3·46 | 3·70 | 3·50 | |||||||||||
| P2O5 | 0·92 | 0·80 | 0·87 | 0·66 | 0·46 | 0·63 | 0·46 | 0·74 | 0·95 | 0·92 | 0·78 | |||||||||||
| LOI | 1·04 | 1·53 | 0·48 | 0·39 | 0·48 | 0·48 | 0·23 | 0·23 | 2·24 | 1·94 | 1·13 | |||||||||||
| Total | 99·67 | 99·83 | 99·69 | 99·69 | 99·92 | 99·92 | 99·59 | 99·59 | 100·22 | 100·01 | 99·92 | |||||||||||
| Q | ||||||||||||||||||||||
| Hy | ||||||||||||||||||||||
| Ne + Lc | 17·2 | 10·0 | 13·9 | 9·9 | 7·2 | 9·5 | 3·5 | 5·4 | 19·3 | 18·6 | 23·8 | |||||||||||
| Rb | 37·5 | 49·0 | 51·0 | 56·0 | 44·5 | 60·6 | 27·5 | 42·5 | 115·0 | 102·0 | 103·0 | |||||||||||
| Sr | 850 | 1000 | 1590 | 1080 | 750 | 1013 | 560 | 866 | 1470 | 1380 | 1030 | |||||||||||
| Ba | 660 | 730 | 760 | 680 | 515 | 706 | 380 | 595 | 1070 | 1200 | 915 | |||||||||||
| Sc | 23·0 | 17·0 | 6·5 | 11·0 | 22·0 | 22·5 | 2·7 | 4·0 | 10·0 | |||||||||||||
| V | 500 | 310 | 282 | 242 | 270 | 230 | 215 | 195 | 262 | |||||||||||||
| Cr | 170 | 59 | 12 | 10 | 480 | 580 | 1 | 1 | 6 | |||||||||||||
| Co | 55 | 36 | 31 | 29 | 50 | 64 | 17 | 21 | 24 | |||||||||||||
| Ni | 120 | 72 | 21 | 50 | 238 | 407 | 1 | 2 | 17 | |||||||||||||
| Y | 38·0 | 36·0 | 28·0 | 34·0 | 26·5 | 32·5 | 22·0 | 30·3 | 39·0 | 34·0 | 37·5 | |||||||||||
| Zr | 270 | 330 | 255 | 365 | 278 | 215 | 382 | 296 | 405 | |||||||||||||
| Nb | 57·0 | 75·0 | 61·0 | 60·0 | 44·0 | 60·7 | 34·0 | 53·9 | 93·0 | 81·0 | 93·0 | |||||||||||
| La | 42·0 | 57·0 | 54·0 | 64·0 | 45·0 | 61·3 | 34·5 | 53·7 | 78·0 | 70·0 | 69·5 | |||||||||||
| Ce | 100·0 | 120·0 | 109·0 | 128·0 | 96·0 | 128·7 | 74·0 | 113·5 | 165·0 | 142·0 | 140·0 | |||||||||||
| Nd | 66·0 | 60·5 | 53·0 | 65·0 | 50·0 | 62·4 | 41·0 | 57·7 | 79·0 | 67·0 | 70·0 | |||||||||||
| Sm | 13·00 | 11·80 | 9·75 | 11·80 | 9·60 | 11·98 | 8·20 | 11·54 | 13·80 | 11·60 | 12·20 | |||||||||||
| Eu | 3·95 | 3·53 | 3·21 | 3·67 | 2·83 | 3·53 | 2·35 | 3·29 | 3·95 | 3·38 | 3·60 | |||||||||||
| Gd | 11·40 | 10·20 | 8·10 | 10·20 | 8·20 | 10·05 | 7·00 | 9·65 | 11·00 | 9·75 | 10·50 | |||||||||||
| Dy | 8·30 | 7·50 | 5·80 | 7·00 | 5·70 | 6·99 | 4·90 | 6·69 | 8·00 | 6·80 | 7·60 | |||||||||||
| Er | 3·40 | 3·20 | 2·55 | 2·90 | 2·40 | 2·94 | 2·00 | 2·76 | 3·40 | 3·10 | 3·30 | |||||||||||
| Yb | 2·25 | 2·44 | 2·03 | 2·03 | 1·67 | 2·05 | 1·38 | 1·90 | 2·67 | 2·35 | 2·61 | |||||||||||
| Th | 3·15 | 7·45 | 6·10 | 8·80 | 5·90 | 8·11 | 4·45 | 6·99 | 10·40 | 6·80 | 9·15 | |||||||||||
| 87Sr/86Sr | 0·704147 ± 10 | 0·705257 ± 9 | 0·705143 ± 8 | 0·703963 ± 6 | 0·703971 ± 9 | |||||||||||||||||
| 143Nd/144Nd | 0·512884 ± 8 | 0·512718 ± 2 | 0·512729 ± 2 | 0·512883 ± 2 | 0·512892 ± 10 | |||||||||||||||||
| Island: . | Tahiti . | Tahiti . | Tahiti . | Tahiti . | Tahiti . | Tahiti . | Tahiti . | Tahiti . | Tahiti . | Raiatea . | Raiatea . | Raiatea . | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample: . | THG-11A . | THG-11A* . | THG-3A . | THG-3A* . | THG-4 . | THG-6A . | THG-3B . | THG-19 . | THG-1Db . | RIG-3B . | RI-28 . | RIG-1B . | ||||||||||||
| Type: . | Ess . | Ess . | Ess . | Ess . | Ne-Msye . | Ne-Msye . | Ne-Sye . | Ne-Sye . | Ne-Sye . | Gab . | Gab . | The . | ||||||||||||
| Group: . | 1 . | 1 . | 1 . | 1 . | 1 . | 1 . | 1 . | 1 . | 1 . | . | . | . | ||||||||||||
| Reference: . | a . | . | a . | . | a . | a . | a . | a . | a . | a . | a, e . | a . | ||||||||||||
| SiO2 | 42·40 | 44·43 | 42·30 | 44·74 | 49·00 | 48·00 | 50·70 | 52·30 | 54·00 | 44·30 | 44·50 | 47·20 | ||||||||||||
| TiO2 | 3·59 | 2·79 | 3·65 | 2·81 | 2·01 | 2·29 | 1·55 | 1·15 | 1·42 | 6·30 | 6·05 | 3·04 | ||||||||||||
| Al2O3 | 15·35 | 18·52 | 15·00 | 18·99 | 19·05 | 18·60 | 20·45 | 20·30 | 18·60 | 15·00 | 14·43 | 18·50 | ||||||||||||
| Fe2O3t | 12·70 | 9·74 | 13·10 | 9·81 | 7·42 | 8·85 | 5·42 | 4·48 | 6·90 | 12·40 | 12·77 | 10·45 | ||||||||||||
| MnO | 0·20 | 0·11 | 0·22 | 0·12 | 0·16 | 0·19 | 0·13 | 0·16 | 0·12 | 0·15 | 0·15 | 0·15 | ||||||||||||
| MgO | 5·88 | 4·15 | 6·25 | 4·19 | 2·78 | 3·05 | 1·22 | 0·89 | 1·21 | 12·00 | 11·50 | 8·10 | ||||||||||||
| CaO | 10·70 | 8·54 | 11·30 | 8·49 | 6·13 | 6·45 | 4·95 | 3·70 | 3·12 | 12·00 | 11·50 | 8·10 | ||||||||||||
| Na2O | 4·45 | 5·81 | 3·90 | 5·46 | 6·40 | 6·20 | 5·95 | 7·00 | 6·52 | 2·80 | 2·82 | 5·00 | ||||||||||||
| K2O | 2·63 | 3·52 | 1·84 | 2·67 | 3·88 | 3·95 | 4·88 | 6·10 | 6·70 | 1·05 | 1·18 | 3·10 | ||||||||||||
| P2O5 | 0·85 | 1·14 | 0·64 | 0·93 | 0·56 | 0·53 | 0·28 | 0·21 | 0·34 | 0·38 | 0·40 | 1·00 | ||||||||||||
| LOI | 1·08 | 1·08 | 1·20 | 1·20 | 2·37 | 1·80 | 4·34 | 3·63 | 1·09 | 0·25 | 0·32 | 0·44 | ||||||||||||
| Total | 99·83 | 99·83 | 99·40 | 99·40 | 99·76 | 99·91 | 99·87 | 99·92 | 100·02 | 100·23 | 99·86 | 100·03 | ||||||||||||
| Q | ||||||||||||||||||||||||
| Hy | ||||||||||||||||||||||||
| Ne + Lc | 18·8 | 23·1 | 14·1 | 17·6 | 18·6 | 19·8 | 14·7 | 21·5 | 17·3 | 3·2 | 2·8 | 12·9 | ||||||||||||
| Rb | 75·5 | 99·3 | 44·0 | 61·5 | 121·0 | 123·0 | 152·0 | 205·0 | 155·0 | 20·5 | 26·0 | 73·0 | ||||||||||||
| Sr | 905 | 1157 | 1035 | 1391 | 1020 | 1010 | 1465 | 890 | 495 | 870 | 800 | 1230 | ||||||||||||
| Ba | 740 | 979 | 720 | 1013 | 1140 | 1050 | 1350 | 1270 | 1050 | 285 | 302 | 740 | ||||||||||||
| Sc | 17·8 | 21·0 | 5·8 | 6·8 | 0·8 | 0·4 | 2·5 | 25·0 | 25·5 | 6·0 | ||||||||||||||
| V | 333 | 320 | 145 | 158 | 82 | 55 | 28 | 425 | 430 | 140 | ||||||||||||||
| Cr | 63 | 102 | 30 | 26 | 1 | 3 | 2 | 54 | 65 | 7 | ||||||||||||||
| Co | 39 | 40 | 17 | 21 | 6 | 4 | 9 | 40 | 42 | 25 | ||||||||||||||
| Ni | 55 | 61 | 17 | 22 | 2 | 2 | 4 | 95 | 97 | 25 | ||||||||||||||
| Y | 34·8 | 38·6 | 34·0 | 38·1 | 28·5 | 30·0 | 32·0 | 26·0 | 46·0 | 22·5 | 25·0 | 36·5 | ||||||||||||
| Zr | 350 | 315 | 280 | 270 | 335 | 495 | 770 | 235 | 250 | 380 | ||||||||||||||
| Nb | 70·0 | 86·0 | 73·0 | 94·0 | 89·0 | 95·0 | 103·0 | 120·0 | 134·0 | 37·0 | 36·0 | 66·0 | ||||||||||||
| La | 56·0 | 71·5 | 57·0 | 76·7 | 65·0 | 69·0 | 85·0 | 73·0 | 106·0 | 25·0 | 27·0 | 62·0 | ||||||||||||
| Ce | 118·0 | 146·8 | 117·0 | 152·1 | 124·0 | 132·0 | 148·0 | 133·0 | 210·0 | 58·0 | 63·0 | 130·0 | ||||||||||||
| Nd | 60·5 | 68·7 | 58·0 | 66·9 | 50·0 | 55·0 | 62·0 | 45·0 | 86·0 | 34·0 | 41·0 | 69·0 | ||||||||||||
| Sm | 11·50 | 13·05 | 11·20 | 12·91 | 8·90 | 9·50 | 10·00 | 7·10 | 14·70 | 7·50 | 10·80 | 13·20 | ||||||||||||
| Eu | 3·30 | 3·84 | 3·20 | 3·80 | 2·63 | 2·75 | 2·96 | 2·00 | 3·17 | 2·50 | 2·55 | 4·02 | ||||||||||||
| Gd | 9·90 | 10·99 | 9·75 | 10·93 | 7·00 | 7·60 | 7·80 | 5·60 | 10·80 | 6·85 | 7·70 | 11·10 | ||||||||||||
| Dy | 7·30 | 8·11 | 7·10 | 7·96 | 5·50 | 5·70 | 5·90 | 4·50 | 8·70 | 4·80 | 5·20 | 7·80 | ||||||||||||
| Er | 3·20 | 3·55 | 3·20 | 3·58 | 2·35 | 3·00 | 2·90 | 2·40 | 4·00 | 1·90 | 2·00 | 3·10 | ||||||||||||
| Yb | 2·38 | 2·62 | 2·59 | 2·87 | 2·10 | 2·39 | 2·23 | 2·23 | 3·40 | 1·40 | 1·50 | 2·24 | ||||||||||||
| Th | 6·15 | 7·99 | 5·41 | 7·45 | 9·60 | 8·80 | 9·90 | 14·00 | 19·40 | 2·55 | 2·70 | 7·60 | ||||||||||||
| 87Sr/86Sr | 0·703965 ± 9 | 0·703937 ± 8 | 0·703958 ± 9 | 0·704433 ± 10 | 0·704434 ± 6 | 0·704367 ± 10 | ||||||||||||||||||
| 143Nd/144Nd | 0·512880 ± 2 | 0·512886 ± 5 | 0·512887 ± 1 | 0·512819 ± 10 | 0·512823 ± 3 | 0·512823 ± 9 | ||||||||||||||||||
| Island: . | Tahiti . | Tahiti . | Tahiti . | Tahiti . | Tahiti . | Tahiti . | Tahiti . | Tahiti . | Tahiti . | Raiatea . | Raiatea . | Raiatea . | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample: . | THG-11A . | THG-11A* . | THG-3A . | THG-3A* . | THG-4 . | THG-6A . | THG-3B . | THG-19 . | THG-1Db . | RIG-3B . | RI-28 . | RIG-1B . | ||||||||||||
| Type: . | Ess . | Ess . | Ess . | Ess . | Ne-Msye . | Ne-Msye . | Ne-Sye . | Ne-Sye . | Ne-Sye . | Gab . | Gab . | The . | ||||||||||||
| Group: . | 1 . | 1 . | 1 . | 1 . | 1 . | 1 . | 1 . | 1 . | 1 . | . | . | . | ||||||||||||
| Reference: . | a . | . | a . | . | a . | a . | a . | a . | a . | a . | a, e . | a . | ||||||||||||
| SiO2 | 42·40 | 44·43 | 42·30 | 44·74 | 49·00 | 48·00 | 50·70 | 52·30 | 54·00 | 44·30 | 44·50 | 47·20 | ||||||||||||
| TiO2 | 3·59 | 2·79 | 3·65 | 2·81 | 2·01 | 2·29 | 1·55 | 1·15 | 1·42 | 6·30 | 6·05 | 3·04 | ||||||||||||
| Al2O3 | 15·35 | 18·52 | 15·00 | 18·99 | 19·05 | 18·60 | 20·45 | 20·30 | 18·60 | 15·00 | 14·43 | 18·50 | ||||||||||||
| Fe2O3t | 12·70 | 9·74 | 13·10 | 9·81 | 7·42 | 8·85 | 5·42 | 4·48 | 6·90 | 12·40 | 12·77 | 10·45 | ||||||||||||
| MnO | 0·20 | 0·11 | 0·22 | 0·12 | 0·16 | 0·19 | 0·13 | 0·16 | 0·12 | 0·15 | 0·15 | 0·15 | ||||||||||||
| MgO | 5·88 | 4·15 | 6·25 | 4·19 | 2·78 | 3·05 | 1·22 | 0·89 | 1·21 | 12·00 | 11·50 | 8·10 | ||||||||||||
| CaO | 10·70 | 8·54 | 11·30 | 8·49 | 6·13 | 6·45 | 4·95 | 3·70 | 3·12 | 12·00 | 11·50 | 8·10 | ||||||||||||
| Na2O | 4·45 | 5·81 | 3·90 | 5·46 | 6·40 | 6·20 | 5·95 | 7·00 | 6·52 | 2·80 | 2·82 | 5·00 | ||||||||||||
| K2O | 2·63 | 3·52 | 1·84 | 2·67 | 3·88 | 3·95 | 4·88 | 6·10 | 6·70 | 1·05 | 1·18 | 3·10 | ||||||||||||
| P2O5 | 0·85 | 1·14 | 0·64 | 0·93 | 0·56 | 0·53 | 0·28 | 0·21 | 0·34 | 0·38 | 0·40 | 1·00 | ||||||||||||
| LOI | 1·08 | 1·08 | 1·20 | 1·20 | 2·37 | 1·80 | 4·34 | 3·63 | 1·09 | 0·25 | 0·32 | 0·44 | ||||||||||||
| Total | 99·83 | 99·83 | 99·40 | 99·40 | 99·76 | 99·91 | 99·87 | 99·92 | 100·02 | 100·23 | 99·86 | 100·03 | ||||||||||||
| Q | ||||||||||||||||||||||||
| Hy | ||||||||||||||||||||||||
| Ne + Lc | 18·8 | 23·1 | 14·1 | 17·6 | 18·6 | 19·8 | 14·7 | 21·5 | 17·3 | 3·2 | 2·8 | 12·9 | ||||||||||||
| Rb | 75·5 | 99·3 | 44·0 | 61·5 | 121·0 | 123·0 | 152·0 | 205·0 | 155·0 | 20·5 | 26·0 | 73·0 | ||||||||||||
| Sr | 905 | 1157 | 1035 | 1391 | 1020 | 1010 | 1465 | 890 | 495 | 870 | 800 | 1230 | ||||||||||||
| Ba | 740 | 979 | 720 | 1013 | 1140 | 1050 | 1350 | 1270 | 1050 | 285 | 302 | 740 | ||||||||||||
| Sc | 17·8 | 21·0 | 5·8 | 6·8 | 0·8 | 0·4 | 2·5 | 25·0 | 25·5 | 6·0 | ||||||||||||||
| V | 333 | 320 | 145 | 158 | 82 | 55 | 28 | 425 | 430 | 140 | ||||||||||||||
| Cr | 63 | 102 | 30 | 26 | 1 | 3 | 2 | 54 | 65 | 7 | ||||||||||||||
| Co | 39 | 40 | 17 | 21 | 6 | 4 | 9 | 40 | 42 | 25 | ||||||||||||||
| Ni | 55 | 61 | 17 | 22 | 2 | 2 | 4 | 95 | 97 | 25 | ||||||||||||||
| Y | 34·8 | 38·6 | 34·0 | 38·1 | 28·5 | 30·0 | 32·0 | 26·0 | 46·0 | 22·5 | 25·0 | 36·5 | ||||||||||||
| Zr | 350 | 315 | 280 | 270 | 335 | 495 | 770 | 235 | 250 | 380 | ||||||||||||||
| Nb | 70·0 | 86·0 | 73·0 | 94·0 | 89·0 | 95·0 | 103·0 | 120·0 | 134·0 | 37·0 | 36·0 | 66·0 | ||||||||||||
| La | 56·0 | 71·5 | 57·0 | 76·7 | 65·0 | 69·0 | 85·0 | 73·0 | 106·0 | 25·0 | 27·0 | 62·0 | ||||||||||||
| Ce | 118·0 | 146·8 | 117·0 | 152·1 | 124·0 | 132·0 | 148·0 | 133·0 | 210·0 | 58·0 | 63·0 | 130·0 | ||||||||||||
| Nd | 60·5 | 68·7 | 58·0 | 66·9 | 50·0 | 55·0 | 62·0 | 45·0 | 86·0 | 34·0 | 41·0 | 69·0 | ||||||||||||
| Sm | 11·50 | 13·05 | 11·20 | 12·91 | 8·90 | 9·50 | 10·00 | 7·10 | 14·70 | 7·50 | 10·80 | 13·20 | ||||||||||||
| Eu | 3·30 | 3·84 | 3·20 | 3·80 | 2·63 | 2·75 | 2·96 | 2·00 | 3·17 | 2·50 | 2·55 | 4·02 | ||||||||||||
| Gd | 9·90 | 10·99 | 9·75 | 10·93 | 7·00 | 7·60 | 7·80 | 5·60 | 10·80 | 6·85 | 7·70 | 11·10 | ||||||||||||
| Dy | 7·30 | 8·11 | 7·10 | 7·96 | 5·50 | 5·70 | 5·90 | 4·50 | 8·70 | 4·80 | 5·20 | 7·80 | ||||||||||||
| Er | 3·20 | 3·55 | 3·20 | 3·58 | 2·35 | 3·00 | 2·90 | 2·40 | 4·00 | 1·90 | 2·00 | 3·10 | ||||||||||||
| Yb | 2·38 | 2·62 | 2·59 | 2·87 | 2·10 | 2·39 | 2·23 | 2·23 | 3·40 | 1·40 | 1·50 | 2·24 | ||||||||||||
| Th | 6·15 | 7·99 | 5·41 | 7·45 | 9·60 | 8·80 | 9·90 | 14·00 | 19·40 | 2·55 | 2·70 | 7·60 | ||||||||||||
| 87Sr/86Sr | 0·703965 ± 9 | 0·703937 ± 8 | 0·703958 ± 9 | 0·704433 ± 10 | 0·704434 ± 6 | 0·704367 ± 10 | ||||||||||||||||||
| 143Nd/144Nd | 0·512880 ± 2 | 0·512886 ± 5 | 0·512887 ± 1 | 0·512819 ± 10 | 0·512823 ± 3 | 0·512823 ± 9 | ||||||||||||||||||
| Island: . | Raiatea . | Raiatea . | Raiatea . | Raiatea . | Raiatea . | Raiatea . | Raiatea . | Raiatea . | Raiatea . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample: . | RIG-1E . | RIG-2A . | RIG-2C . | RIG-2D . | RIG-4A . | RIG-4B . | RIG-4C . | RI-85 . | RI-86 . | |||||||||
| Type: . | The . | The . | The . | The . | The . | The . | The . | The . | The . | |||||||||
| Group: . | . | . | . | . | . | . | . | . | . | |||||||||
| Reference: . | a . | a . | a . | a . | a . | a . | a . | a, e . | a, e . | |||||||||
| SiO2 | 44·60 | 47·60 | 47·00 | 46·80 | 46·40 | 47·30 | 42·00 | 45·80 | 46·60 | |||||||||
| TiO2 | 4·24 | 2·94 | 3·18 | 3·30 | 4·19 | 3·30 | 5·34 | 3·62 | 3·34 | |||||||||
| Al2O3 | 17·45 | 18·60 | 17·80 | 17·90 | 16·60 | 16·80 | 13·70 | 17·08 | 18·35 | |||||||||
| Fe2O3t | 12·30 | 10·00 | 10·20 | 10·85 | 11·90 | 11·25 | 15·30 | 11·18 | 10·75 | |||||||||
| MnO | 0·15 | 0·14 | 0·15 | 0·15 | 0·15 | 0·16 | 0·19 | 0·16 | 0·14 | |||||||||
| MgO | 9·90 | 8·50 | 8·70 | 8·60 | 3·58 | 4·08 | 6·40 | 3·33 | 3·17 | |||||||||
| CaO | 9·90 | 8·50 | 8·70 | 8·60 | 9·55 | 8·10 | 10·00 | 10·00 | 9·72 | |||||||||
| Na2O | 4·30 | 5·15 | 5·00 | 4·58 | 4·50 | 4·80 | 3·70 | 4·09 | 4·25 | |||||||||
| K2O | 1·95 | 2·60 | 2·75 | 3·20 | 2·20 | 2·48 | 1·58 | 2·35 | 2·23 | |||||||||
| P2O5 | 1·05 | 1·00 | 1·15 | 0·99 | 0·80 | 0·83 | 0·80 | 1·12 | 0·82 | |||||||||
| LOI | 0·21 | 0·52 | 0·84 | 0·44 | 0·04 | 0·76 | 0·70 | 1·14 | 0·91 | |||||||||
| Total | 100·03 | 99·93 | 99·92 | 99·96 | 99·91 | 99·86 | 99·71 | 99·87 | 100·28 | |||||||||
| Q | ||||||||||||||||||
| Hy | ||||||||||||||||||
| Ne + Lc | 10·6 | 11·9 | 12·2 | 12·0 | 10·0 | 9·9 | 10·6 | 8·8 | 8·8 | |||||||||
| Rb | 37·5 | 53·0 | 60·0 | 85·5 | 47·0 | 65·0 | 31·5 | 52·0 | 50·0 | |||||||||
| Sr | 1160 | 1250 | 1220 | 1170 | 970 | 1100 | 1020 | 1165 | 1190 | |||||||||
| Ba | 560 | 730 | 715 | 690 | 530 | 700 | 430 | 642 | 585 | |||||||||
| Sc | 10·8 | 6·0 | 7·5 | 7·3 | 11·2 | 9·5 | 13·0 | 10·0 | 8·8 | |||||||||
| V | 255 | 135 | 138 | 155 | 285 | 225 | 365 | 154 | 175 | |||||||||
| Cr | 10 | 6 | 5 | 30 | 2 | 38 | 95 | 7 | 10 | |||||||||
| Co | 34 | 22 | 22 | 24 | 27 | 30 | 49 | 25 | 26 | |||||||||
| Ni | 38 | 20 | 20 | 23 | 38 | 50 | 127 | 18 | 23 | |||||||||
| Y | 33·0 | 36·5 | 37·0 | 38·0 | 39·5 | 34·0 | 31·0 | 40·0 | 34·0 | |||||||||
| Zr | 335 | 370 | 376 | 370 | 386 | 420 | 365 | 363 | 340 | |||||||||
| Nb | 55·0 | 64·0 | 64·0 | 66·0 | 60·0 | 79·0 | 66·0 | 67·0 | 58·0 | |||||||||
| La | 52·0 | 62·5 | 62·0 | 60·0 | 54·0 | 65·0 | 52·0 | 61·0 | 52·5 | |||||||||
| Ce | 115·0 | 132·0 | 133·0 | 130·0 | 119·0 | 136·0 | 111·0 | 133·0 | 110·0 | |||||||||
| Nd | 62·0 | 67·5 | 69·0 | 67·0 | 66·0 | 65·0 | 62·5 | 76·0 | 63·0 | |||||||||
| Sm | 12·4 | 13·10 | 13·80 | 13·20 | 13·60 | 12·30 | 12·20 | 17·10 | 13·10 | |||||||||
| Eu | 3·72 | 3·93 | 4·12 | 3·92 | 4·00 | 3·70 | 3·67 | 4·25 | 3·50 | |||||||||
| Gd | 10·75 | 11·10 | 11·90 | 11·20 | 11·50 | 10·30 | 10·10 | 12·80 | 10·60 | |||||||||
| Dy | 7·40 | 7·60 | 7·80 | 7·80 | 8·20 | 7·05 | 7·10 | 8·30 | 6·90 | |||||||||
| Er | 2·80 | 3·00 | 3·10 | 3·00 | 3·20 | 2·90 | 2·80 | 3·50 | 2·80 | |||||||||
| Yb | 2·03 | 2·25 | 2·27 | 2·29 | 2·44 | 2·18 | 1·76 | 2·35 | 2·10 | |||||||||
| Th | 6·00 | 7·95 | 7·65 | 7·80 | 6·10 | 8·70 | 6·50 | 6·80 | 6·70 | |||||||||
| 87Sr/86Sr | 0·704085 ± 5 | |||||||||||||||||
| 143Nd/144Nd | 0·512853 ± 2 | |||||||||||||||||
| Island: . | Raiatea . | Raiatea . | Raiatea . | Raiatea . | Raiatea . | Raiatea . | Raiatea . | Raiatea . | Raiatea . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample: . | RIG-1E . | RIG-2A . | RIG-2C . | RIG-2D . | RIG-4A . | RIG-4B . | RIG-4C . | RI-85 . | RI-86 . | |||||||||
| Type: . | The . | The . | The . | The . | The . | The . | The . | The . | The . | |||||||||
| Group: . | . | . | . | . | . | . | . | . | . | |||||||||
| Reference: . | a . | a . | a . | a . | a . | a . | a . | a, e . | a, e . | |||||||||
| SiO2 | 44·60 | 47·60 | 47·00 | 46·80 | 46·40 | 47·30 | 42·00 | 45·80 | 46·60 | |||||||||
| TiO2 | 4·24 | 2·94 | 3·18 | 3·30 | 4·19 | 3·30 | 5·34 | 3·62 | 3·34 | |||||||||
| Al2O3 | 17·45 | 18·60 | 17·80 | 17·90 | 16·60 | 16·80 | 13·70 | 17·08 | 18·35 | |||||||||
| Fe2O3t | 12·30 | 10·00 | 10·20 | 10·85 | 11·90 | 11·25 | 15·30 | 11·18 | 10·75 | |||||||||
| MnO | 0·15 | 0·14 | 0·15 | 0·15 | 0·15 | 0·16 | 0·19 | 0·16 | 0·14 | |||||||||
| MgO | 9·90 | 8·50 | 8·70 | 8·60 | 3·58 | 4·08 | 6·40 | 3·33 | 3·17 | |||||||||
| CaO | 9·90 | 8·50 | 8·70 | 8·60 | 9·55 | 8·10 | 10·00 | 10·00 | 9·72 | |||||||||
| Na2O | 4·30 | 5·15 | 5·00 | 4·58 | 4·50 | 4·80 | 3·70 | 4·09 | 4·25 | |||||||||
| K2O | 1·95 | 2·60 | 2·75 | 3·20 | 2·20 | 2·48 | 1·58 | 2·35 | 2·23 | |||||||||
| P2O5 | 1·05 | 1·00 | 1·15 | 0·99 | 0·80 | 0·83 | 0·80 | 1·12 | 0·82 | |||||||||
| LOI | 0·21 | 0·52 | 0·84 | 0·44 | 0·04 | 0·76 | 0·70 | 1·14 | 0·91 | |||||||||
| Total | 100·03 | 99·93 | 99·92 | 99·96 | 99·91 | 99·86 | 99·71 | 99·87 | 100·28 | |||||||||
| Q | ||||||||||||||||||
| Hy | ||||||||||||||||||
| Ne + Lc | 10·6 | 11·9 | 12·2 | 12·0 | 10·0 | 9·9 | 10·6 | 8·8 | 8·8 | |||||||||
| Rb | 37·5 | 53·0 | 60·0 | 85·5 | 47·0 | 65·0 | 31·5 | 52·0 | 50·0 | |||||||||
| Sr | 1160 | 1250 | 1220 | 1170 | 970 | 1100 | 1020 | 1165 | 1190 | |||||||||
| Ba | 560 | 730 | 715 | 690 | 530 | 700 | 430 | 642 | 585 | |||||||||
| Sc | 10·8 | 6·0 | 7·5 | 7·3 | 11·2 | 9·5 | 13·0 | 10·0 | 8·8 | |||||||||
| V | 255 | 135 | 138 | 155 | 285 | 225 | 365 | 154 | 175 | |||||||||
| Cr | 10 | 6 | 5 | 30 | 2 | 38 | 95 | 7 | 10 | |||||||||
| Co | 34 | 22 | 22 | 24 | 27 | 30 | 49 | 25 | 26 | |||||||||
| Ni | 38 | 20 | 20 | 23 | 38 | 50 | 127 | 18 | 23 | |||||||||
| Y | 33·0 | 36·5 | 37·0 | 38·0 | 39·5 | 34·0 | 31·0 | 40·0 | 34·0 | |||||||||
| Zr | 335 | 370 | 376 | 370 | 386 | 420 | 365 | 363 | 340 | |||||||||
| Nb | 55·0 | 64·0 | 64·0 | 66·0 | 60·0 | 79·0 | 66·0 | 67·0 | 58·0 | |||||||||
| La | 52·0 | 62·5 | 62·0 | 60·0 | 54·0 | 65·0 | 52·0 | 61·0 | 52·5 | |||||||||
| Ce | 115·0 | 132·0 | 133·0 | 130·0 | 119·0 | 136·0 | 111·0 | 133·0 | 110·0 | |||||||||
| Nd | 62·0 | 67·5 | 69·0 | 67·0 | 66·0 | 65·0 | 62·5 | 76·0 | 63·0 | |||||||||
| Sm | 12·4 | 13·10 | 13·80 | 13·20 | 13·60 | 12·30 | 12·20 | 17·10 | 13·10 | |||||||||
| Eu | 3·72 | 3·93 | 4·12 | 3·92 | 4·00 | 3·70 | 3·67 | 4·25 | 3·50 | |||||||||
| Gd | 10·75 | 11·10 | 11·90 | 11·20 | 11·50 | 10·30 | 10·10 | 12·80 | 10·60 | |||||||||
| Dy | 7·40 | 7·60 | 7·80 | 7·80 | 8·20 | 7·05 | 7·10 | 8·30 | 6·90 | |||||||||
| Er | 2·80 | 3·00 | 3·10 | 3·00 | 3·20 | 2·90 | 2·80 | 3·50 | 2·80 | |||||||||
| Yb | 2·03 | 2·25 | 2·27 | 2·29 | 2·44 | 2·18 | 1·76 | 2·35 | 2·10 | |||||||||
| Th | 6·00 | 7·95 | 7·65 | 7·80 | 6·10 | 8·70 | 6·50 | 6·80 | 6·70 | |||||||||
| 87Sr/86Sr | 0·704085 ± 5 | |||||||||||||||||
| 143Nd/144Nd | 0·512853 ± 2 | |||||||||||||||||
Accumulation-free chemical compositions estimated by using the MONA program (see text for explanations).
Abbreviations as in Table 1. Q, Hy, Ne, and Lc are CIPW normative mineral proportions calculated with Fe2O3/FeO varying with the petrographic type (Middlemost, 1989). Group 1 corresponds to the strongly Si-undersaturated suite, Group 2 to the mildly Si-undersaturated suite, and Group 3 includes strongly Si-undersaturated rocks with low 87Sr/86Sr and high 143Nd/144Nd. References: a, this work; b, Clément et al. (2002); c, Bardintzeff et al. (1988); d, Nitecki-Novotni (1975); e, Blais et al. (1997) (major elements).
Loss on ignition and major element variations
All samples have loss on ignition (LOI) values lower than 2·5 wt %, except the three coarse-grained rocks THG-3B, -10B, and -19 (Table 4). These latter rocks display obvious alteration features: bowlingitization of olivine and damouritization of plagioclase in clinopyroxenite THG-10B, cancrinization of nepheline and/or kaolinization of K-feldspar in ne-syenites THG-3B and -19.
The presence or the lack of nepheline in the coarse-grained rocks (including ultrabasites) finds expression in their normative foid contents (ne + lc), calculated following the CIPW procedure, with Fe3+/Fe2+ ratios assumed to be dependent on the degree of differentiation (Middlemost, 1989). The nepheline-free rocks, together with ol-clinopyroxenites, are mildly silica-undersaturated rocks (ne + lc <6·5 wt %), except the two monzonites that contain normative hypersthene (Table 4). The other rocks, including cpx-hornblendites, are strongly silica undersaturated, even if theralite THG-1A is less rich in normative nepheline (ne = 3·5 wt %), as a result of its cumulative characteristics.
Both groups defined on the basis of normative compositions can be also distinguished using the TAS diagram (Fig. 8). For instance, among the ultrabasites, the cpx-hornblendites are shifted towards the low-SiO2 side, whereas the slightly more siliceous ol-clinopyroxenites are less alkaline. For the whole sample set, alkalinity is strongly coupled with the degree of Si undersaturation.
Total alkalis–silica (TAS) discrimination diagram for coarse-grained rocks from the Ahititera and Faaroa depressions. The dotted lines indicate the effects of corrections to the bulk-rock compositions of the moderately cumulative rocks to take account of the cumulus phases (Table 4). The curve divides the strongly Si-undersaturated rocks from the mildly Si-undersaturated rocks. Circled symbols are Raiatea samples.
Selected major element concentrations are presented vs MgO in Fig. 9 for the Ahititera rocks. All samples having MgO concentrations ≥10 wt % are either ultrabasites (clinopyroxenites) or moderately cumulative rocks (theralites). The latter samples have SiO2, CaO and TiO2 concentrations fairly similar to those of their non-cumulative theralitic equivalents, but they are shifted towards high MgO values as a result of their abundant accumulated olivine and clinopyroxene. With decreasing MgO (from 10 wt %), concentrations of SiO2 and Al2O3 increase roughly whereas those of CaO and TiO2 decrease, irrespective of the level of silica undersaturation. Concentrations of P2O5 are more scattered. However, the lowest values correspond to those of the mildly Si-undersaturated rocks, with the exception of the monzonite TP6. Noteworthy features are the particularly high P2O5 content of the apatite-rich hornblendite THG-2D (1·90 wt %) and the low value for gabbro THG-10E, also characterized by a high TiO2 concentration.
Selected major oxides (wt %) and trace elements (ppm) vs MgO and Th, respectively, for the Ahititera samples. The dashed field delineates the bulk-rock compositions of the nepheline-free rocks in the P2O5 vs MgO diagram. The dotted lines connect the bulk-rock compositions of the moderately cumulative rocks to their corresponding corrected values (Table 4). No accumulation correction for Ni. Symbols as in Fig. 8.
Trace element and isotopic variations
Representative REE patterns normalized to C1 chondrites (Sun & McDonough, 1989) are shown in Fig. 10. All the coarse-grained rocks from Tahiti Nui and Raiatea are enriched in light rare earth elements (LREE) with respect to heavy rare earth elements (HREE), a feature characteristic of alkaline ocean island magmas. Ol-clinopyroxenite THG-10B has the lowest REE concentrations and shows the flattest pattern. The other ultrabasite, cpx-hornblendite THG-2D, has high middle REE (MREE) values. Theralites and gabbros from the two islands display comparable HREE concentrations but contrasted LREE concentrations; LREE are more enriched in the most Si-undersaturated rocks (theralites). The syenites have concave-up REE patterns, broadly symmetrical with the hornblendite pattern.
C1 chondrite-normalized REE patterns of representative samples from Ahititera and Faaroa [normalization values from Sun & McDonough (1989)]. Circled symbols are Raiatea samples.
For the Ahititera plutonic samples, Ni, Sr, Nb, La, and Yb are plotted vs Th, regarded as the most incompatible element (Fig. 9). With increasing Th, Ni concentrations display a hyperbolic decreasing trend, except for the two moderately cumulative theralites THG-1A and -2A. Sr and Yb concentrations are very scattered, contrasting with those of Nb and La, which show good positive correlations with Th.
Clément et al. (2002) presented the first Sr and Nd isotopic data ever published on Society Islands coarse-grained rocks (three Ahititera plutonic rocks: THG-9E1, -10C, -13A). For this study, 13 additional coarse-grained rocks from Tahiti Nui and Raiatea have been analysed for the same isotopes (Table 4). The data are shown in the 143Nd/144Nd vs 87Sr/86Sr diagram of Fig. 11, together with fields for Society seamounts and Raiatea and Tahiti Nui volcanoes. Our new data from Raiatea and Tahiti Nui plot within the previously defined fields for both islands. As for other ocean island suites, lavas from the Society Archipelago define an array with negative slope in the 143Nd/144Nd vs 87Sr/86Sr diagram (e.g. White, 1985). This array, extending between the Depleted MORB-Mantle (DMM) and Enriched Mantle II (EMII) end-members, is almost identical to that of the Society seamounts shown in Fig. 11.
143Nd/144Nd vs 87Sr/86Sr diagram showing data for Ahititera and Faaroa rocks, compared with lavas of the Society Archipelago (Tahiti Nui field: White & Hofmann, 1982; Cheng et al., 1993; Hémond et al., 1994; Le Roy, 1994; White & Duncan, 1996; Raiatea field: C. Chauvel, personal communication, 2003; Society seamounts: Devey et al., 1990; Hémond et al., 1994). The isotope compositions of a clast and a dyke collected from Ahititera are also shown (Clément et al., 2002). The mantle end-members HIMU, DMM, EMI, and EMII are from Hart & Zindler (1989). Chronological quadrants defined for Tahitian lavas by Le Roy (1994): the least radiogenic lavas (plotting into quadrant 1) are dated between 600 and 180 ka, whereas the most radiogenic lavas (quadrant 2) have ages ranging from 1400 to 800 ka. The number of samples within each petrographic type is indicated in parentheses for Group 1. Symbols as in Fig. 8.
Isotope compositions of the Ahititera pluton split into three groups. The first (nine strongly Si-undersaturated samples) plots close to the most depleted edge of the Tahitian trend. The two theralites THG-1A and -2B (Group 3) plot near the most enriched end of the trend. Group 2 is composed of three mildly Si-undersaturated rocks having intermediate Nd–Sr isotope compositions. Among the lavas analysed by Clément et al. (2002), a tephritic clast is related to Group 1 whereas a trachybasaltic dyke plots near Group 2. Both gabbros and one theralite from Raiatea have similar Nd–Sr isotope compositions, intermediate between those of Groups 1 and 2 of Ahititera, whereas theralite RIG-4C is slightly shifted towards the depleted end of the Raiatea field.
A TEXTURAL CLASSIFICATION
A first textural distinction we can make concerns the presence or lack of cumulus crystals, despite the fact that they are often difficult to identify. Coarse-grained rocks sampled by drilling, or as xenoliths in lavas, are often regarded as cumulates by petrologists (e.g. Augé et al., 1989; Rançon et al., 1989; Hoover & Fodor, 1997). Such a generalization does not apply to our sample set, which can be divided into three groups: cumulates (>50% cumulus phases), moderately cumulative rocks (between 20 and 50% cumulus phases), and non-cumulative rocks.
Cumulates
These rocks are generally made up a framework of ‘cumulus crystals’, also called ‘primocrysts’, which are typically subhedral to euhedral. They are cemented together by a texturally later generation of ‘intercumulus’ crystals (Wager & Brown, 1968). Although the formation of cumulate rocks is complex and follows various mechanisms, none of them can be considered to be the result of simple in situ crystallization of a magmatic liquid. Their nomenclature is based on the crystallization modalities and on the textural relations between cumulus and intercumulus phases (Wager et al., 1960; Irvine, 1982). Among our set of coarse-grained rocks from Tahiti Nui, only the ultrabasic rocks are cumulates. Ol-clinopyroxenites contain more than 75 vol. % cumulus phases (diopside and olivine), and cpx-hornblendites more than 60 vol. % cumulus phases (mainly kaersutite and diopside). Following the classification of Irvine (1982), the first group displays a mesocumulative texture. The second group has an orthocumulate texture, with, locally, heteradcumulate patches (i.e. cumulus crystals are included within larger intercumulus minerals).
Non-cumulative and moderately cumulative rocks
The rocks free of accumulated crystals represent the most abundant textural type. They may be classified in terms of equigranular, intergranular and oikocrystic textural end-members. We propose in Fig. 12 two simple quantitative parameters, the relative values of which can be used to make distinction between these textural types. Parameter Li (in mm) is defined as the maximum length measured in the analysed area for the mineral species i. The crystal density Di (number of crystals per mm2) is equal to Ni/si, where Ni is the number of grains of phase i in the analysed area and si (in mm2) the area covered by mineral phase i. The phases i considered are the main minerals of the rocks: clinopyroxene, feldspars and/or nepheline.
Triangular textural classification for non-cumulative, coarse-grained rocks. Each textural end-member is characterized by specific relative values of two parameters measured on a phase i: crystal density (Di) and maximum crystal length (Li). The D and L triangular cursors are plotted on an arbitrary axis having a length proportional to the highest values measured for the corresponding parameters. For instance, for an axis length corresponding to Li = 2·2 mm (feldspar), the Li triangular cursor of pyroxene (value of 2·1 mm) is adjusted between 0 and 2·2 mm. The typical textures are illustrated by sketches, together with photomicrographs (crossed nicols) for the end-members. The dotted fields include the textures observed in the different petrographic groups defined in the Ahititera and Faaroa plutons.
An equigranular texture consists of crystals in contact with each other, through a side-by-side relationship (Hibbard, 1995). The main constitutive minerals are characterized by nearly similar lengths. The grain interfaces can be simply planar or more complex. Inclusions are generally lacking. Within our sample set, this end-member is represented by the texture of gabbro THG-10C (Lcpx/Lplg = 1·1 and Dcpx/Dplg = 2·4).
Non-cumulative magmatic rocks exhibit an intergranular texture when a part of the rock-forming minerals fills isolated spaces between the coarser touching grains that form the framework of the rock. If some of these voids are filled with glassy patches, the texture is then termed intersertal. The corresponding texture for foid-bearing intermediate or differentiated rocks is named foyaitic (Sørensen, 1974), and is represented in our set by ne-syenite THG-3B. This sample is characterized by contrasted Li and Di values: feldspar laths are longer than interstitial nepheline crystals; nepheline crystal density is higher than Dfeld (Dneph/Dfeld = 3·5).
The oikocrystic texture is mainly characterized by the systematic occurrence of including grain interrelations. It consists of grains or laths included or partially included in larger and typically anhedral crystals (Hibbard, 1995). This texture is named ‘ophitic’ if crystals of pyroxene or amphibole include plagioclase laths. It is represented by theralite RIG-2A. In terms of parameters, this sample also shows contrasted Li and Di values, but here the included feldspars are smaller and have a higher crystal density than the host clinopyroxenes (Dcpx/Dplg = 0·2).
These textural end-members represent the three apices of the triangle in Fig. 12, which can be used to classify all non-cumulative (and non-pegmatoid) coarse-grained rocks. Transitions between intergranular and equigranular textures, mainly characterized by the lack of including grain relations, are named ‘heterogranular textures’. Textures intermediate between equigranular and oikocrystic end-members are named ‘granular textures with including relations’. Finally, the ‘doleritic textures’ correspond to the transition between intergranular and oikocrystic end-members.
Textural features of non-cumulative rocks are related to their petrography: gabbros and theralites show granular textures with (or without) including relations, whereas all the nepheline-bearing intermediate and differentiated rocks plot near the intergranular–foyaitic end-member, even if there are, in some essexites, patches where crystals show incipient pegmatoid characteristics (high elongation, acicular and skeletal shapes). Monzonites exhibit unequivocal pegmatoid features (Bardintzeff et al., 1988), and alkali syenites are characterized by an isogranular secondary texture, as a result of a recrystallization process.
In the moderately cumulative rocks, similar distinctions can be made from the coarse-grained groundmass, the cumulative fraction being considered as a simple textural overprint.
CRYSTALLIZATION AND FLUID TRANSFER IN AHITITERA PLUTON
Late and post-magmatic processes
Replacement of clinopyroxene
Partial replacement of clinopyroxene by hornblende occurs only in the strongly Si-undersaturated rocks, especially in essexites and ne-monzosyenites. These late amphiboles appear either as peripheral brown flakes associated with small magnetite grains (Fig. 5d) or as more or less thick, inclusion-free, rims. In some essexites, the presence of scarce clinopyroxene relicts in the central part of euhedral hornblendes might indicate the almost total replacement of primary clinopyroxenes by amphiboles. Generally, such secondary amphiboles, poor in Ti and rich in alkali elements, are pargasitic or hastingsitic in composition (Giret et al., 1980; Gillis & Meyer, 2001). In the Ahititera samples, most of the replacement amphiboles are kaersutite, like the cores of the primary hornblendes (Table 3, Fig. 7a). Their late crystallization is only suggested by their position in the (AlIV + Ca) vs (Si + Na + K) diagram of Fig. 7b. These sub-solidus transformations denote either a hydrothermal alteration (400–800°C: Agemar et al., 1999) or a deuteric process (800–900°C: Tribuzio et al., 2000).
CO2-related alteration
Calcite and ankerite have been observed only in the strongly Si-undersaturated rocks and are especially abundant in the ne-monzosyenites. They fill diktytaxitic voids or occur as interstitial phases. In the carbonate-rich rocks, nepheline crystals are commonly partially converted into cancrinite. This feature can be interpreted as the result of reaction between early nepheline crystals and CO2-bearing residual liquids (Deer et al., 1992).
Such late or post-magmatic features provide evidence for release of CO2-saturated fluids at the end of the crystallization course of the strongly Si-undersaturated rocks.
Fe–Ti oxides
In the mafic rocks (ol-clinopyroxenites, gabbros, and theralites), Fe–Ti oxides are generally mantled by biotite, more rarely by kaersutite, as the result of their reaction with alkali-rich residual liquids. Red biotite in contact with haemoilmenite is richer in titanium (TiO2 > 8·5 wt %) than the brown biotite that rims titanomagnetite (TiO2 < 9·5 wt %).
Titanomagnetite contains, almost systematically, lamellae of haemoilmenite, indicative of exsolution–oxidation phenomena. Such processes are generally interpreted as resulting from high-temperature, subsolidus reorganization (>600°C) in slow cooling conditions further to a PH2O increase (Mathison, 1975).
Secondary Fe–Ti oxides are also observed in association with chlorite and clay minerals (bowlingite-type alteration), fringing olivine and filling fractures within olivine. This association results from a low-temperature alteration process (c. 200°C, Schandl et al., 1990).
Mineral accumulation
Cumulates
The textural study has shown that cumulates sampled from the Ahititera pluton consist of a framework of cumulus crystals cemented with intercumulus phases, the composition of which probably reflects that of interstitial liquids. If this assumption is justified for the orthocumulative cpx-hornblendite, it is only an approximation for the mesocumulative ol-clinopyroxenite. Each cumulate type has been included into a group having equivalent Si undersaturation, as a function of its modal and normative nepheline contents (Figs 4 and 8). These links are confirmed by the position of cumulates in the Nd–Sr isotopic diagram of Fig. 11. Thus, the composition of interstitial liquids might be approximated by that of non-cumulative rocks having comparable Si undersaturation.
We have reconstructed the modal composition of clinopyroxenite THG-10B by introducing in a least-squares mass balance program (MOde Near Analysis MONA program: Metzner & Grimmeisen, 1990) the major element concentrations of the corresponding whole-rock (Table 4), its cumulus clinopyroxene and olivine (electron microprobe analyses), and a non-cumulative rock regarded as representative of the interstitial liquid. The latter rock was selected from the gabbros, based on the primary mineralogy of the intercumulus material of THG-10B (clinopyroxene, olivine, plagioclase, Fe–Ti oxides and apatite). The result is presented in Table 5 (using THG-10C as a proxy for the interstitial liquid: 30·3 wt %). This provides a statistically good fit, as the sum of the squares of the residuals (ΣR2) equals 0·79. Contrary to the modal proportions (wt %), which show that clinopyroxene is the principal mafic phase in the bulk-rock (Table 2), the calculated percentages of cumulus phases indicate olivine and clinopyroxene in sub-equal proportions (Table 5). We then reconstructed the trace element composition of the modelled cumulate. The result is shown as a Primitive Mantle-normalized trace element variation diagram in Fig. 13. The calculated and measured patterns are nearly identical, except for Rb, Ba, and Sr; this is probably due to the set of distribution coefficients used (see Appendix).
Trace element patterns normalized to Primitive Mantle (from Sun & McDonough, 1989) for analysed and reconstructed Ahititera cumulates (using the distribution coefficients in the Appendix). Symbols as in Fig. 8. (See text for details.)
MONA (MOde Near Analysis) models for the Ahititera cumulates
| Cumulate . | L . | f . | Weight percentages of cumulus phases . | ΣR2 . |
|---|---|---|---|---|
| Ol-Cpxite | Gab THG-10C | 30·3 | 36·6 Ol + 32·4 Cpx | 0·79 |
| Cpx-Hblite | Ess THG-2C | 25·5 | 36·2 Hbl + 29·8 Cpx + 7·6 Fe–Ti Ox + 1·2 Ap | 0·72 |
| Ne-Msye THG-4 | 20·6 | 40·8 Hbl + 28·8 Cpx + 8·2 Fe–Ti Ox + 1·9 Ap | 0·92 |
| Cumulate . | L . | f . | Weight percentages of cumulus phases . | ΣR2 . |
|---|---|---|---|---|
| Ol-Cpxite | Gab THG-10C | 30·3 | 36·6 Ol + 32·4 Cpx | 0·79 |
| Cpx-Hblite | Ess THG-2C | 25·5 | 36·2 Hbl + 29·8 Cpx + 7·6 Fe–Ti Ox + 1·2 Ap | 0·72 |
| Ne-Msye THG-4 | 20·6 | 40·8 Hbl + 28·8 Cpx + 8·2 Fe–Ti Ox + 1·9 Ap | 0·92 |
f, weight percentage of intercumulus material, the composition of which is approached by the rock L, inferred to represent a liquid. ΣR2, sum of the squared residuals. For cpx-hornblendite, our preferred model is that implying the ne-monzosyenite THG-4. Abbreviations as in Table 1 and as follows: Ol, olivine; Cpx, clinopyroxene; Hbl, hornblende; Fe–Ti Ox, Fe–Ti oxides, Ap, apatite.
MONA (MOde Near Analysis) models for the Ahititera cumulates
| Cumulate . | L . | f . | Weight percentages of cumulus phases . | ΣR2 . |
|---|---|---|---|---|
| Ol-Cpxite | Gab THG-10C | 30·3 | 36·6 Ol + 32·4 Cpx | 0·79 |
| Cpx-Hblite | Ess THG-2C | 25·5 | 36·2 Hbl + 29·8 Cpx + 7·6 Fe–Ti Ox + 1·2 Ap | 0·72 |
| Ne-Msye THG-4 | 20·6 | 40·8 Hbl + 28·8 Cpx + 8·2 Fe–Ti Ox + 1·9 Ap | 0·92 |
| Cumulate . | L . | f . | Weight percentages of cumulus phases . | ΣR2 . |
|---|---|---|---|---|
| Ol-Cpxite | Gab THG-10C | 30·3 | 36·6 Ol + 32·4 Cpx | 0·79 |
| Cpx-Hblite | Ess THG-2C | 25·5 | 36·2 Hbl + 29·8 Cpx + 7·6 Fe–Ti Ox + 1·2 Ap | 0·72 |
| Ne-Msye THG-4 | 20·6 | 40·8 Hbl + 28·8 Cpx + 8·2 Fe–Ti Ox + 1·9 Ap | 0·92 |
f, weight percentage of intercumulus material, the composition of which is approached by the rock L, inferred to represent a liquid. ΣR2, sum of the squared residuals. For cpx-hornblendite, our preferred model is that implying the ne-monzosyenite THG-4. Abbreviations as in Table 1 and as follows: Ol, olivine; Cpx, clinopyroxene; Hbl, hornblende; Fe–Ti Ox, Fe–Ti oxides, Ap, apatite.
A similar procedure was carried out for the cpx-hornblendite THG-14. In this rock, accumulation of amphibole, a mineral displaying high MREE distribution coefficients (Caroff et al., 1999), might explain the high MREE contents observed for this group in Fig. 10. The other cumulus phases are diopside, Fe–Ti oxides, and apatite. The composition of the interstitial liquid could be either essexitic or ne-monzosyenitic, given the mineralogy of the intercumulus material (in order of decreasing abundance: plagioclase, clinopyroxene, Fe–Ti oxides, apatite, nepheline, titanite, and K-feldspar). Two model calculations were carried out, with essexite THG-2C and ne-monzosyenite THG-4 as interstitial liquids. The relative mineral percentages in the cumulus assemblages are similar in the two cases, but the proportions of interstitial liquid are slightly different (25·5 wt % for essexite and 20·6 wt % for ne-monzosyenite, Table 5). Both models reproduce adequately the major element composition of THG-14 (ΣR2 <1, Table 5); however, the trace element data lead us clearly to prefer the model based on the ne-monzosyenite composition (Fig. 13).
Correction of bulk-rock compositions for crystal accumulation in the moderately cumulative rocks
Among our set of non-ultrabasic samples, five display some petrographic and geochemical evidence for mineral accumulation (Tables 2 and 4, Fig. 9): gabbro THG-10E (high CaO, TiO2, Sr, and Nb values; low P2O5 values); theralites THG-1A and -2A, and essexites THG-3A and -11A (high MgO and compatible trace elements).
The petrographic characteristics of gabbro THG-10E suggest that its chemical peculiarities can be explained by accumulation of amphibole ± Fe–Ti oxides (high TiO2 and Nb) and plagioclase (high CaO and Sr), coupled with apatite fractionation (low P2O5). The concomitant intervention of two opposite processes (accumulation and fractionation) involving several minerals makes any correction impossible for this rock.
Theralite THG-1A belongs to Group 3 defined in the Nd–Sr isotope diagram of Fig. 11. Group 3, characterized by high Th/Nb values, also includes THG-2A, a sample not analysed for isotopes. Petrographic study of THG-1A and -2A has revealed the presence of numerous large, euhedral crystals of clinopyroxene and olivine (Table 2). Accumulation of these minerals might account for high MgO, Sc, Cr, Co, and Ni contents in both theralites. The bulk-rock compositions of these samples have been corrected assuming that the moderately cumulative rocks are a combination of minerals accumulated within a theralitic liquid. The calculation method is based on a MONA modal reconstruction involving three phases (liquid + accumulated olivine + accumulated clinopyroxene). The liquid composition (major elements) has been approximated in both cases by that of a Group 3 non-cumulative theralite (THG-2B). Once the phase proportions have been determined (Table 2: THG-1A′ and -2A′), a simple mass balance calculation is used to remove the chemical contribution of accumulated minerals in THG-1A and -2A. The results are shown in Table 4 (corrected compositions: THG-1A* and -2A*). The correction shifts the two samples towards the theralitic group in the TAS diagram of Fig. 8, and within the main evolutionary trends in the variation diagrams of Fig. 9.
A similar procedure has been followed for essexites THG-3A and -11A, using the major element composition of non-cumulative essexite THG-2C as the liquid phase. Here, the accumulated minerals are barely identifiable from textural criteria alone, except for the presence of zoned euhedral crystals of clinopyroxene in THG-11A and xenocrystic olivine (Table 2). In addition, the two cumulative rocks, although chemically very similar (high MgO, Sc, Cr, Co, and Ni contents: Table 4), are clearly distinguishable by their total proportions of clinopyroxene (9·7 wt % and 29·0 wt % for THG-3A and -11A, respectively, Table 2) and amphibole (41·1 wt % and 19·3 wt %). However, textural relationships suggest that most of the observed amphiboles in THG-3A correspond to transformed accumulated clinopyroxenes. This is corroborated by the bulk-rock REE contents; these are similar in both the amphibole-rich sample (THG-3A) and the amphibole-poor sample (THG-11A), in which clinopyroxene is clearly the main cumulus phase. Such similarity is not consistent with the accumulation of magmatic amphibole, a mineral containing large amounts of MREE. Thus, we have calculated theoretical modes for both rocks, including a liquid phase, accumulated olivine, clinopyroxene and Fe–Ti oxides, but devoid of cumulus amphibole (Table 2: THG-3A′ and -11A′). The accumulation-free chemical compositions are shown in Table 4 (THG-3A* and -11A*). As for the theralites, this correction reduces the scatter observed within the essexitic group in Figs 8 and 9.
Evolution of the two magmatic suites
A number of geological, mineralogical and geochemical features suggest the occurrence of two isotopically contrasted suites of magmatic rocks (plus the isolated theralitic Group 3), which are mildly and strongly Si undersaturated, respectively. Each suite includes a range of petrographic types from basic to felsic and is isotopically homogeneous. The strongly Si-undersaturated suite (Group 1) includes three clinopyroxene-hornblendites, two theralites, five essexites, two nepheline-monzosyenites and three nepheline-syenites; the mildly Si-undersaturated suite (Group 2) includes two olivine-clinopyroxenites, five gabbros, two monzonites and one alkali syenite (Table 4).
To constrain the nature and the proportions of the fractionating phases within each suite, we have combined the major element concentrations of representative non-cumulative samples together with microprobe analyses of their main euhedral minerals into a least-squares fractionation (LSF) program (Bryan et al., 1969; Wright & Doherty, 1970). This method is used here to determine the most ‘reasonable’ proportions of minerals separating from the liquid at each stage. Then, these data are combined with individual mineral–melt distribution coefficients (see Appendix) to estimate the trace element composition of the daughter liquid, based on the trace element concentrations in the parental magma, the fraction of residual liquid, and the bulk distribution coefficients, for a Rayleigh fractionation model.
The mildly Si-undersaturated suite
The mildly Si-undersaturated suite is not well documented as only four petrographic types have been collected. In addition, the gabbros are chemically heterogeneous (e.g. Fig. 9), the monzonites are known only through previous studies (Nitecki-Novotny, 1975; Bardintzeff et al., 1988), and the alkali syenite is hydrothermally altered.
From gabbros to monzonites. The chosen parental liquid is gabbro THG-10C, which has been analysed for Nd–Sr isotopes. The daughter is monzonite 37H (Nitecki-Novotny, 1975; Bardintzeff et al., 1988). The proportions of fractionating minerals and residual liquid are shown in Table 6 together with the sum of the squares of the residuals (ΣR2). The fractionating assemblage is mainly composed of clinopyroxene, plagioclase and hornblende (in decreasing order). The percentage of residual liquid is 38·8 wt %. In the alkali–silica diagram of Fig. 14a, the compositions of the analysed parental and daughter samples are plotted together with the calculated daughter liquid, which plots close to the analysed sample, and the calculated fractionating assemblage (LSF: least-squares fractionation). The calculated trace element composition of the daughter liquid, shown as a normalized pattern in Fig. 15a, is similar to that of monzonite 37H, except for Eu (minor plagioclase accumulation?) and Yb.
TAS diagrams showing the results of the fractionation/accumulation models for the mildly Si-undersaturated (a) and strongly Si-undersaturated (b) suites from Ahititera. LSF-derived liquids and fractionating assemblages are theoretical compositions calculated by a least-squares fractionation method (see text for details). The arrow from THG-13A to THG-2C denotes a fractionation stage under hydrous conditions, which induces alkali enrichment from theralites to essexites (see text for further explanation).
Ahititera trace element patterns normalized to Primitive Mantle (from Sun & McDonough, 1989) for inferred parental and daughter melts (analysed and/or modelled). The trace element compositions of theoretical liquids have been calculated by using the LSF parameters and the Rayleigh equation. All the parent magma compositions correspond to analytical data, except that of the monzonite (see text). The calculated daughter liquids are illustrated and compared with the analysed samples. Inset: comparison between (analysed) Group 1 theralites and non-cumulative (plus corrected) essexites.
LSF (least-squares fractionation) models for the two Ahititera suites
| Parental magmas . | Fractionated magmas . | F . | Weight percentages of fractionating assemblages . | ΣR2 . | ||||
|---|---|---|---|---|---|---|---|---|
| Mildly Si-undersaturated suite | ||||||||
| Gab THG-10C | Monz 37H | 38·8 | 40·7 Cpx + 23·3 Plg + 23·3 Hbl + 7·6 Fe–Ti Ox + 4·6 Ol + 0·5 Ap | 0·44 | ||||
| Monz 37H | Alk-Sye THG-9B | 46·4 | 39·8 Hbl + 32·7 Plg + 12·7 Fe–Ti Ox + 12·1 Cpx + 2·8 Ap | 4·53 | ||||
| Strongly Si-undersaturated suite | ||||||||
| Ess THG-2C | Ne-Msye THG-4 | 67·4 | 53·3 Hbl + 24·1 Plg + 8·9 Fe–Ti Ox + 8·2 Ne + 5·5 Ap | 0·77 | ||||
| Ne-Msye THG-4 | Ne-Sye THG-3B | 66·6 | 34·5 Cpx + 27·0 Plg + 26·9 Ne + 11·0 Fe–Ti Ox + 0·6 Ap | 1·02 | ||||
| Parental magmas . | Fractionated magmas . | F . | Weight percentages of fractionating assemblages . | ΣR2 . | ||||
|---|---|---|---|---|---|---|---|---|
| Mildly Si-undersaturated suite | ||||||||
| Gab THG-10C | Monz 37H | 38·8 | 40·7 Cpx + 23·3 Plg + 23·3 Hbl + 7·6 Fe–Ti Ox + 4·6 Ol + 0·5 Ap | 0·44 | ||||
| Monz 37H | Alk-Sye THG-9B | 46·4 | 39·8 Hbl + 32·7 Plg + 12·7 Fe–Ti Ox + 12·1 Cpx + 2·8 Ap | 4·53 | ||||
| Strongly Si-undersaturated suite | ||||||||
| Ess THG-2C | Ne-Msye THG-4 | 67·4 | 53·3 Hbl + 24·1 Plg + 8·9 Fe–Ti Ox + 8·2 Ne + 5·5 Ap | 0·77 | ||||
| Ne-Msye THG-4 | Ne-Sye THG-3B | 66·6 | 34·5 Cpx + 27·0 Plg + 26·9 Ne + 11·0 Fe–Ti Ox + 0·6 Ap | 1·02 | ||||
LSF (least-squares fractionation) models for the two Ahititera suites
| Parental magmas . | Fractionated magmas . | F . | Weight percentages of fractionating assemblages . | ΣR2 . | ||||
|---|---|---|---|---|---|---|---|---|
| Mildly Si-undersaturated suite | ||||||||
| Gab THG-10C | Monz 37H | 38·8 | 40·7 Cpx + 23·3 Plg + 23·3 Hbl + 7·6 Fe–Ti Ox + 4·6 Ol + 0·5 Ap | 0·44 | ||||
| Monz 37H | Alk-Sye THG-9B | 46·4 | 39·8 Hbl + 32·7 Plg + 12·7 Fe–Ti Ox + 12·1 Cpx + 2·8 Ap | 4·53 | ||||
| Strongly Si-undersaturated suite | ||||||||
| Ess THG-2C | Ne-Msye THG-4 | 67·4 | 53·3 Hbl + 24·1 Plg + 8·9 Fe–Ti Ox + 8·2 Ne + 5·5 Ap | 0·77 | ||||
| Ne-Msye THG-4 | Ne-Sye THG-3B | 66·6 | 34·5 Cpx + 27·0 Plg + 26·9 Ne + 11·0 Fe–Ti Ox + 0·6 Ap | 1·02 | ||||
| Parental magmas . | Fractionated magmas . | F . | Weight percentages of fractionating assemblages . | ΣR2 . | ||||
|---|---|---|---|---|---|---|---|---|
| Mildly Si-undersaturated suite | ||||||||
| Gab THG-10C | Monz 37H | 38·8 | 40·7 Cpx + 23·3 Plg + 23·3 Hbl + 7·6 Fe–Ti Ox + 4·6 Ol + 0·5 Ap | 0·44 | ||||
| Monz 37H | Alk-Sye THG-9B | 46·4 | 39·8 Hbl + 32·7 Plg + 12·7 Fe–Ti Ox + 12·1 Cpx + 2·8 Ap | 4·53 | ||||
| Strongly Si-undersaturated suite | ||||||||
| Ess THG-2C | Ne-Msye THG-4 | 67·4 | 53·3 Hbl + 24·1 Plg + 8·9 Fe–Ti Ox + 8·2 Ne + 5·5 Ap | 0·77 | ||||
| Ne-Msye THG-4 | Ne-Sye THG-3B | 66·6 | 34·5 Cpx + 27·0 Plg + 26·9 Ne + 11·0 Fe–Ti Ox + 0·6 Ap | 1·02 | ||||
Role of the ol-clinopyroxenites in the fractional crystallization model. We have reported in Fig. 14a the analysed ol-clinopyroxenite THG-10B, the calculated cumulate (MONA), and the LSF fractionating assemblage. The latter plots between the ol-clinopyroxenites (THG-10B and its MONA reconstruction) and the gabbro THG-10C. We have shown that the ol-clinopyroxenite THG-10B is a hybrid cumulate comprising c. 30 wt % crystallized gabbroic liquid. Its low alkali content can be explained by its mafic cumulus mineralogy (Table 5), which might reflect a fractionating assemblage formed before the gabbro–monzonite stage. These results are not consistent with the model of Bardintzeff et al. (1988), who interpreted the ol-clinopyroxenites as an equivalent of the assemblage fractionated during the gabbro–monzonite transition.
From monzonites to alkali syenites. This stage of magmatic evolution is difficult to model because: (1) the monzonite composition is known only from previous studies; (2) the alkali syenite is hydrothermally altered; (3) there is a significant chemical gap between these two rocks. Given the restricted trace element analyses for the monzonites, the parental liquid has been taken to be equal to the calculated residual liquid of the previous fractionation stage (from gabbro to monzonite, Fig. 15a). The LSF model of Table 6, if not well constrained from a statistical point of view (ΣR2 = 4·5), reproduces correctly the trace element composition of the postulated derived liquid (Fig. 15b). The high alkali contents in the analysed sample (with reference to the calculated liquid) might reflect an alkali enrichment in the alkali syenite as a result of alteration. The position of the corresponding LSF fractionating assemblage is shifted towards low alkali and silica values, as a result of the excessive magnitude of the differentiation step.
The strongly Si-undersaturated suite
From Group 1 theralites to essexites. Fractionating assemblages resulting from LSF calculations (not shown) require fractionation of Fe–Ti oxides in amounts (>18 wt %) larger than those usually considered in closed-system fractional crystallization models. In addition, the alkali increase from theralites to essexites is not coupled with any MgO decrease (Fig. 16a). In addition, Rb increases abruptly from c. 50 ppm in theralites to c. 100 ppm in essexites, whereas the other incompatible trace elements increase moderately (Table 4). Thus, the essexites cannot be derived from theralites through a simple process. The resemblance of the REE patterns of both groups (Fig. 15c inset), together with their similar isotope compositions, suggests, however, that there is a link between them. In addition to the alkali enrichment, any evolutionary mechanism must also account for the chemical and mineralogical features listed below.
Chemical evidence for differentiation under hydrous conditions from Group 1 theralites to essexites (Ahititera). (a) Na2O + K2O vs MgO (wt %) diagram showing the alkali increase from theralites to essexites at constant MgO. (b) Na2O + K2O–FeOt–MgO (AFM) diagram for the strongly Si-undersaturated suite. The dashed line represents the ‘normal’ evolution trend. The transition from theralites to essexites is marked by an abrupt FeOt decrease, probably related to significant fractionation of Fe–Ti oxides under high fO2 conditions. Symbols as in Fig. 8.
(1) In the AFM (alkali–FeOt–MgO) diagram of Fig. 16b, the transition from theralites to essexites is marked by an abrupt FeOt decrease, which might be related to significant fractionation of Fe–Ti oxides, as already suggested by LSF calculations. Such a hypothesis has already been proposed by Brandriss & Bird (1999) to explain a similar shift in composition of evolved rocks from the Kap Edvard Holm Gabbroic Complex, Greenland. Following Brandriss & Bird, fractionation of abundant Fe–Ti oxides reflects crystallization under more hydrous and/or more oxidizing conditions than those typical of alkali magmas (see also Sisson & Grove, 1993; Ghiorso, 1997).
(2) The essexites THG-2C and THG-11B (not analysed) contain pegmatoid patches. Such features, also observed by Brandriss & Bird (1999) in their FeOt-depleted rocks, are generally interpreted as indicators of crystallization under hydrous conditions (Larsen & Brooks, 1994; Mitchell et al., 1997).
(3) All the essexites are characterized by the presence of abundant early euhedral hornblende crystals (Table 2, Fig. 4), a mineral crystallizing under high water pressures.
(4) Clinopyroxene and plagioclase display Ca and Fe3+ enrichment from theralites to essexites (Table 3, Fig. 17). Increase of Ca in plagioclase is generally interpreted as the result of a rise of the water pressure in the magma (Yoder et al., 1957; Tepley et al., 2000). The Fe3+ content is a marker of the fO2: oxidizing conditions favour its incorporation within plagioclase and clinopyroxene structures (Marcelot et al., 1988; Phinney, 1992).
Mineral chemical evidence for differentiation under hydrous conditions from Group 1 theralites to essexites (Ahititera). (a) Fe3+ vs Ca (cations per formula unit) in cores of clinopyroxene for theralites (triangles) and essexites (stars). (b) Fe3+ vs Ca (cations per formula unit) in plagioclase (Ab <90; Or <3).
All these features strongly suggest that the transition from theralites to essexites was accompanied by an increase of PH2O and fO2, implying an opening of the magmatic system, with influx of H2O and alkali elements. This inference prevents any quantitative modelling of the transition between theralites to essexites.
From essexites to ne-syenites through to ne-monzosyenites. These three petrographic groups are isotopically homogeneous (Fig. 11). Two LSF stages have been modelled with low ΣR2 values (Table 6, Fig. 14b). The trace element reconstructions are good (except for the slightly high Rb, Ba and Th values in both theoretical liquids and underestimation of Sr in the calculated ne-syenite, Fig. 15c and d). The essexite–ne-monzosyenite transition implies mostly the separation of hornblende and plagioclase. During the following stage, the main fractionating minerals were clinopyroxene, plagioclase, and nepheline. The percentage of residual liquid is c. 67 wt % in the two cases (Table 6).
Place of the cpx-hornblendites in the fractional crystallization model. Although cpx-hornblendite THG-14 is a hybrid cumulate (20·6 wt % liquid in the preferred MONA calculation, Table 5), it plots close to the essexite–ne-monzosyenite LSF fractionating assemblage in the TAS diagram of Fig. 14b. The main cumulus mineral of both cpx-hornblendites and essexite–ne-monzosyenite LSF assemblage is kaersutite (Tables 5 and 6). The latter assemblage is devoid of clinopyroxene, but contains plagioclase, a phase observed only in the intercumulus part of the cpx-hornblendites. Thus, the cumulus fraction of the cpx-hornblendites should reflect to some extent the assemblage formed during the essexite–ne-monzosyenite stage.
PARTIAL MELTING OF A CHEMICALLY HETEROGENEOUS MANTLE SOURCE
Mantle source composition
Several volcanoes from the Society Islands, especially Tahiti Nui (Cheng et al., 1993; White & Duncan, 1996), are characterized by a shield stage more radiogenic in Sr and less radiogenic in Nd than the subsequent, more alkaline stage. This isotopic evolution is opposite to that observed in the Marquesas (e.g. Caroff et al., 1995; Le Dez et al., 1996), but identical to that in Hawaii (Chen & Frey, 1985). It is consistent with the zoned mantle plume model proposed, for example, by White & Duncan (1996). First, the EMII-carrying hot central part of the plume produces, through high degrees of melting, the shield lavas. Then, magma is derived by smaller extents of melting of the cooler, isotopically depleted, sheath to the plume to generate the post-shield alkaline lavas.
This model may account for the chemistry of the Tahiti Nui lavas, as the systematic K–Ar dating of Le Roy (1994) has been used to establish a strong intercorrelation between ages, isotope compositions and Si undersaturation of the subaerial lavas: the most recent lavas are both the most Si undersaturated and the most isotopically depleted (Clément et al., 2002; see also Fig. 11). We use our data to extend to the Ahititera pluton, at least for Groups 1 and 2, the correlation between Si undersaturation and Nd–Sr isotope ratios as defined for the Tahitian lavas. Thus, despite the restricted dimensions of the Ahititera pluton, its main geochemical features are in agreement with the zoned plume model proposed to account for chemistry of the Tahitian lavas.
The Group 3 theralites do not follow the same trend. Indeed, despite their high Si-undersaturation index (Table 4) and their REE patterns close to that of other theralites (Fig. 18a), Group 3 theralites plot towards the enriched end of the Tahiti isotopic field. They can also be distinguished from mafic rocks of Groups 1 and 2 by their higher Th/Nb ratios (Fig. 18b). We use this feature to relate another theralitic sample to this group, not analysed for isotopes (THG-2A).
Comparison between gabbros and theralites from the Ahititera pluton. Two of the Group 3 theralites are corrected to take account of mineral accumulation. (a) C1 chondrite-normalized REE patterns [normalization values from Sun & McDonough (1989)]. (b) Plot of Th/Nb vs La/Yb for Ahititera mafic samples.
Th/Nb and Nd–Sr isotope ratios of mafic rocks can be fractionated either by mantle-related processes or by oceanic crustal contamination (Wedepohl, 1995). The latter process is implausible because an isotopic shift from Group 1 to Group 3 would have required incorporation of material rich in incompatible trace elements (e.g. leucocratic rocks). Such assimilation would have modified the shape of the REE patterns, which is not observed in Fig. 18a. In other respects, the Th/Nb ratio is known to be particularly sensitive to the presence in the mantle source of a continental component, supposed to be part of the EMII end-member (White & Hofmann, 1982; White, 1985; Wedepohl, 1995). Derivation of Group 3 theralites from such a mantle, including recycled terrigenous sediments, would account for their shift towards the EMII end-member, as observed in Fig. 11.
The distinctive chemical characteristics of the Group 3 theralites (strong Si undersaturation, steep REE patterns and enriched isotope compositions) have never been observed elsewhere in Tahiti (Cheng et al., 1993; Le Roy, 1994; White & Duncan, 1996). However, such features have already been described in lavas collected from seamounts located SE of Tahiti, in the present-day hotspot area. Indeed, some basanites from Teahitia and Yves Rocard volcanoes plot very close to Group 3 in a Nd–Sr isotopic diagram, whereas other basalts and basanites from the Society seamounts display more depleted isotope compositions (see field in Fig. 11). These data suggest that the Society mantle source is isotopically heterogeneous at various scales. In addition to the large chemical concentric zonation of the plume, expressed during the prolonged build-up of the subaerial lava pile (White & Duncan, 1996), partial melting of local EMII-enriched zones of mantle might explain the chemical characteristics of both Group 3 theralites and the equivalent Teahitia–Rocard basanites.
In Raiatea, the gabbroic and theralitic samples analysed for Nd–Sr isotopes plot within the most depleted part of the Raiatea lava field. Although less scattered than the Tahiti Nui data, the isotope compositions of the Raiatea samples are not consistent with the hypothesis of a homogeneous mantle source. However, the small number of outcrops does not allow us to propose an overall isotopic characterization of the Faaroa pluton.
Partial melting characteristics
Contrasted major element and REE compositions between gabbros and theralites from Tahiti Nui and Raiatea may reflect source heterogeneity, variable degrees of partial melting, and/or differences in the fractional crystallization paths, more or less coupled with contamination. Given the scarcity of REE-bearing primocrysts in the Raiatea and Tahiti Nui gabbros and theralites (Fig. 4), it would be difficult to invoke fractional crystallization to account for the different REE compositions observed between the two mafic rock types (Fig. 19a). The lack of xenoliths and evidence for mineralogical disequilibrium do not provide evidence for a contamination process. Mantle heterogeneities are usually invoked to account for correlations between incompatible trace element and isotope ratios. Alternatively, isotopically homogeneous mafic rocks with variable REE ratios are thought to reflect different degree of partial melting.
Comparison between REE patterns of strongly and mildly Si-undersaturated mafic rocks from the Society Islands [normalization values from Sun & McDonough (1989)]. (a) Fields of mafic coarse-grained rocks (theralites and gabbros) from Tahiti Nui and Raiatea plutons. Theralites THG-1A and -2A from Tahiti Nui are corrected to take account of mineral accumulation. (b) REE patterns of mafic lavas (3 wt % < MgO < 11 wt %: basanites and basalts) from Moua Pihaa, Teahitia, and Yves Rocard seamounts (Dupuy et al., 1989; Hémond et al., 1994). Inset: fields of basanites (n = 22) and basalts (n = 39) from Tahiti Nui and Raiatea islands (for Raiatea: unpublished data, Blais et al., 1997; for Tahiti Nui: unpublished data, Dostal et al., 1982; Hémond et al., 1994; Le Roy, 1994; Clément et al., 2002).
The REE compositions of the mafic coarse-grained rocks from Tahiti Nui and Raiatea are strongly connected to the petrographic types. Although less marked, such a link also exists among the alkali basalts and basanites from both islands (Fig. 19b inset). Isotope ratios display more complex relationships (Fig. 11). First, between Group 1 and 2 from Tahiti Nui, they differ as a function of the petrographic type, together with the REE compositions. Some gabbros and theralites from Raiatea are isotopically homogeneous. Additionally, a few theralites from Raiatea (RIG-4C) and Tahiti Nui (Group 3 samples), although displaying REE compositions similar to those of the other theralites, are isotopically distinct. Mantle heterogeneities, highlighted by such isotopic diversity, cannot readily account for the contrasted REE patterns of the gabbros and theralites. Variable degrees of partial melting of a heterogeneous mantle source are, therefore, required to explain both the Nd–Sr isotope and REE characteristics.
Mantle source heterogeneity beneath both Tahiti Nui and Raiatea prevents quantitative partial melting modelling. However, several studies have shown that basanites result from lower partial melting degrees than alkali basalts (e.g. Edgar, 1987; Caroff et al., 1997). If we extend this assumption to theralites and gabbros, it is possible to discuss the mineralogical characteristics of the melting source by using HREE ratios. Figure 20a and b shows variations of Eu/Dy and Dy/Yb ratios against La in the Raiatea and Tahiti Nui mafic samples. These ratios are rather sensitive indices of garnet- vs clinopyroxene-controlled element fractionation. Indeed, only garnet is able to fractionate HREE during partial melting (Fig. 20b inset). Decreasing degrees of partial melting of a garnet-bearing lherzolite source should result in an increase of Eu/Dy and Dy/Yb ratios (Caroff et al., 1997). As these ratios remain constant from gabbros to theralites, we propose that melting occurred within the spinel-lherzolite facies in both cases.
REE ratios in mafic rocks from Tahiti Nui, Raiatea, and hotspot seamounts from Society. The four Raiatea samples analysed for Nd–Sr isotopes are arrowed. Ranges for basalts and basanites from Moua Pihaa, Teahitia, and Yves Rocard seamounts and from Tahiti Nui and Raiatea are also shown (see references in Fig. 19). (a) Eu/Dy vs La (ppm). (b) Dy/Yb vs La. Inset: distribution coefficient patterns of clinopyroxene and garnet in mantle lherzolites (from Feigenson et al., 1983).
Gabbros and (accumulation-corrected) theralites from the Ahititera and Raiatea plutons can be compared with equivalent lavas (basalts and basanites) collected from hotspot-related seamounts (Figs 19 and 20). Their Nd–Sr isotope compositions span the entire Society field (Fig. 11). It appears that: (1) in each Si-saturation group of samples (gabbro–basalt and theralite–basanite), the REE spectra are broadly sub-parallel, independent of rock texture, Nd–Sr isotope composition, and the island or seamount they come from; (2) the REE patterns of the most Si-undersaturated rocks are systematically steeper (Fig. 19); (3) Eu/Dy and Dy/Yb ratios are broadly constant (Fig. 20). Therefore, REE concentrations in Society mafic rocks are probably mainly governed by the modalities of the partial melting process, irrespective of the isotope signature of the mantle source.
CONCLUSIONS
(1) Most petrological studies of ocean islands rocks deal with lavas, which are generally regarded as crystallized melts. This study is distinct in that we develop a detailed procedure to answer petrogenetic questions using coarse-grained rocks. First, a preliminary textural study must be carried out to quantify the cumulus fraction (often difficult to recognize) in order to focus on the geochemical characteristics of the melt phase. Second, any mineralogical re-equilibration and replacement, together with other late-stage magmatic processes, must be taken into consideration before any modelling.
(2) The two plutons of Faaroa (Raiatea) and Ahititera (Tahiti Nui) are located in the central part of the islands, within horseshoe-shaped calderas. They display a great variety of textural and petrographic types, classified into mildly and strongly Si-undersaturated groups. Clément et al. (2002) have proposed for the Ahititera pluton an emplacement model in which the strongly Si-undersaturated rocks intrude the central part of an older (mildly Si-undersaturated) complex.
(3) The Ahititera samples comprise three isotopic groups. One of them (Group 3), devoid of differentiated members, is atypical (for Tahiti), as it consists of strongly Si-undersaturated rocks with radiogenic Sr isotope ratios. These features appear to reflect local isotopic heterogeneities of the Society mantle plume. In the two other groups, increasing Si undersaturation is clearly correlated with decreasing 87Sr/86Sr, as already documented for Tahitian lavas. These features suggest the probable existence of a large zoned plume. Each of these two groups includes mafic rocks, related cumulates, and their differentiation products. The evolution of the mildly Si-undersaturated suite can be modelled through simple fractional crystallization, whereas the differentiation of the strongly Si-undersaturated suite requires further H2O influx between theralitic and essexitic stages. This influx has been responsible for selective enrichment in alkalis and massive fractionation of Fe–Ti oxides plus amphibole. The strongly Si-undersaturated suite is also characterized by the existence of CO2-saturated fluids at the end of the crystallization.
(4) The REE compositions of the mafic rocks from both the Faaroa and the Ahititera plutons are little dependent on the mantle source composition. REE patterns of the most Si-undersaturated rocks exhibit the steepest slopes. Such features are also observed in lavas from both islands and from hotspot seamounts. Thus, it appears that REE concentrations in Society mafic rocks are probably mainly governed by the modalities of partial melting, irrespective of the isotopic signature of the mantle source. The mantle partially melting underneath both Raiatea and Tahiti is garnet free and partial melting degrees required to produce theralitic melts are lower than those leading to gabbroic ones.
APPENDIX
Mineral–liquid distribution coefficients
| Mineral: . | Ol . | Cpx . | Fe–Ti Ox . | Ne . | Plg . | Ap . | Fe–Ti Ox . | Plg . | Hbl . |
|---|---|---|---|---|---|---|---|---|---|
| Host rock: . | B-HW . | B-HW . | B-HW . | B-HW . | HW . | MG . | MG . | BM . | BM . |
| Reference: . | 1 . | 1 . | 1 . | 2 . | 3 . | 4 . | 3 . | 3 . | 3 . |
| Rb | 0·08 | 0·04 | 0·08 | 0·44 | 0·30 | 0·4 | 0·23 | 0·20 | 0·15 |
| Sr | 0·00 | 0·16 | 0·16 | 0·24 | 2·12 | 4·4 | 0·23 | 4·41 | 1·01 |
| Y | 0·00 | 0·70 | 0·19 | 0·011 | 0·05 | 8 | 0·55 | 0·24 | 2·88 |
| Nb | 0·00 | 0·05 | 1·50 | 0·01 | 0·00 | 0·4 | 1·5 | 0·00 | 0·39 |
| Ba | 0·04 | 0·04 | 0·14 | 0·09 | 0·24 | 0·1 | 0·14 | 1·08 | 0·61 |
| La | 0·02 | 0·10 | 0·20 | 0·01 | 0·12 | 16 | 0·53 | 0·46 | 0·56 |
| Ce | 0·00 | 0·20 | 0·20 | 0·011 | 0·14 | 16 | 0·56 | 0·36 | 0·87 |
| Nd | 0·00 | 0·60 | 0·20 | 0·013 | 0·07 | 18 | 0·55 | 0·31 | 1·82 |
| Sm | 0·00 | 0·60 | 0·20 | 0·012 | 0·12 | 17 | 0·55 | 0·27 | 2·4 |
| Eu | 0·03 | 0·57 | 0·06 | 0·043 | 0·22 | 3·5 | 0·15 | 1·78 | 2·08 |
| Gd | 0·00 | 0·70 | 0·20 | 0·013 | 0·17 | 7 | 0·55 | 0·30 | 2·5 |
| Dy | 0·00 | 0·70 | 0·20 | 0·013 | 0·03 | 2 | 0·55 | 0·23 | 2·8 |
| Er | 0·00 | 0·70 | 0·20 | 0·014 | 0·15 | 3 | 0·6 | 0·23 | 2·5 |
| Yb | 0·00 | 0·70 | 0·20 | 0·016 | 0·11 | 2 | 0·75 | 0·18 | 1·77 |
| Th | 0·04 | 0·03 | 0·18 | 0·014 | 0·02 | 0·95 | 0·53 | 0·16 | 0·09 |
| Mineral: . | Ol . | Cpx . | Fe–Ti Ox . | Ne . | Plg . | Ap . | Fe–Ti Ox . | Plg . | Hbl . |
|---|---|---|---|---|---|---|---|---|---|
| Host rock: . | B-HW . | B-HW . | B-HW . | B-HW . | HW . | MG . | MG . | BM . | BM . |
| Reference: . | 1 . | 1 . | 1 . | 2 . | 3 . | 4 . | 3 . | 3 . | 3 . |
| Rb | 0·08 | 0·04 | 0·08 | 0·44 | 0·30 | 0·4 | 0·23 | 0·20 | 0·15 |
| Sr | 0·00 | 0·16 | 0·16 | 0·24 | 2·12 | 4·4 | 0·23 | 4·41 | 1·01 |
| Y | 0·00 | 0·70 | 0·19 | 0·011 | 0·05 | 8 | 0·55 | 0·24 | 2·88 |
| Nb | 0·00 | 0·05 | 1·50 | 0·01 | 0·00 | 0·4 | 1·5 | 0·00 | 0·39 |
| Ba | 0·04 | 0·04 | 0·14 | 0·09 | 0·24 | 0·1 | 0·14 | 1·08 | 0·61 |
| La | 0·02 | 0·10 | 0·20 | 0·01 | 0·12 | 16 | 0·53 | 0·46 | 0·56 |
| Ce | 0·00 | 0·20 | 0·20 | 0·011 | 0·14 | 16 | 0·56 | 0·36 | 0·87 |
| Nd | 0·00 | 0·60 | 0·20 | 0·013 | 0·07 | 18 | 0·55 | 0·31 | 1·82 |
| Sm | 0·00 | 0·60 | 0·20 | 0·012 | 0·12 | 17 | 0·55 | 0·27 | 2·4 |
| Eu | 0·03 | 0·57 | 0·06 | 0·043 | 0·22 | 3·5 | 0·15 | 1·78 | 2·08 |
| Gd | 0·00 | 0·70 | 0·20 | 0·013 | 0·17 | 7 | 0·55 | 0·30 | 2·5 |
| Dy | 0·00 | 0·70 | 0·20 | 0·013 | 0·03 | 2 | 0·55 | 0·23 | 2·8 |
| Er | 0·00 | 0·70 | 0·20 | 0·014 | 0·15 | 3 | 0·6 | 0·23 | 2·5 |
| Yb | 0·00 | 0·70 | 0·20 | 0·016 | 0·11 | 2 | 0·75 | 0·18 | 1·77 |
| Th | 0·04 | 0·03 | 0·18 | 0·014 | 0·02 | 0·95 | 0·53 | 0·16 | 0·09 |
Ol, olivine; Cpx, clinopyroxene; Fe–Ti Ox, Fe–Ti oxides; Ne, nepheline, Plg, plagioclase, Hbl, hornblende; B, basanite; HW, hawaiite; MG, mugearite; BM, benmoreite. 1, From Villemant et al. (1981) and Lemarchand et al. (1987); 2, from Larsen (1979); 3, from Caroff et al. (1993) except Nb in amphibole (Klein et al., 1997); 4, from Chazot et al. (1996) except Th (Caroff et al., 1993). Values in italics are distribution coefficients extrapolated from those of neighbouring REE.
Mineral–liquid distribution coefficients
| Mineral: . | Ol . | Cpx . | Fe–Ti Ox . | Ne . | Plg . | Ap . | Fe–Ti Ox . | Plg . | Hbl . |
|---|---|---|---|---|---|---|---|---|---|
| Host rock: . | B-HW . | B-HW . | B-HW . | B-HW . | HW . | MG . | MG . | BM . | BM . |
| Reference: . | 1 . | 1 . | 1 . | 2 . | 3 . | 4 . | 3 . | 3 . | 3 . |
| Rb | 0·08 | 0·04 | 0·08 | 0·44 | 0·30 | 0·4 | 0·23 | 0·20 | 0·15 |
| Sr | 0·00 | 0·16 | 0·16 | 0·24 | 2·12 | 4·4 | 0·23 | 4·41 | 1·01 |
| Y | 0·00 | 0·70 | 0·19 | 0·011 | 0·05 | 8 | 0·55 | 0·24 | 2·88 |
| Nb | 0·00 | 0·05 | 1·50 | 0·01 | 0·00 | 0·4 | 1·5 | 0·00 | 0·39 |
| Ba | 0·04 | 0·04 | 0·14 | 0·09 | 0·24 | 0·1 | 0·14 | 1·08 | 0·61 |
| La | 0·02 | 0·10 | 0·20 | 0·01 | 0·12 | 16 | 0·53 | 0·46 | 0·56 |
| Ce | 0·00 | 0·20 | 0·20 | 0·011 | 0·14 | 16 | 0·56 | 0·36 | 0·87 |
| Nd | 0·00 | 0·60 | 0·20 | 0·013 | 0·07 | 18 | 0·55 | 0·31 | 1·82 |
| Sm | 0·00 | 0·60 | 0·20 | 0·012 | 0·12 | 17 | 0·55 | 0·27 | 2·4 |
| Eu | 0·03 | 0·57 | 0·06 | 0·043 | 0·22 | 3·5 | 0·15 | 1·78 | 2·08 |
| Gd | 0·00 | 0·70 | 0·20 | 0·013 | 0·17 | 7 | 0·55 | 0·30 | 2·5 |
| Dy | 0·00 | 0·70 | 0·20 | 0·013 | 0·03 | 2 | 0·55 | 0·23 | 2·8 |
| Er | 0·00 | 0·70 | 0·20 | 0·014 | 0·15 | 3 | 0·6 | 0·23 | 2·5 |
| Yb | 0·00 | 0·70 | 0·20 | 0·016 | 0·11 | 2 | 0·75 | 0·18 | 1·77 |
| Th | 0·04 | 0·03 | 0·18 | 0·014 | 0·02 | 0·95 | 0·53 | 0·16 | 0·09 |
| Mineral: . | Ol . | Cpx . | Fe–Ti Ox . | Ne . | Plg . | Ap . | Fe–Ti Ox . | Plg . | Hbl . |
|---|---|---|---|---|---|---|---|---|---|
| Host rock: . | B-HW . | B-HW . | B-HW . | B-HW . | HW . | MG . | MG . | BM . | BM . |
| Reference: . | 1 . | 1 . | 1 . | 2 . | 3 . | 4 . | 3 . | 3 . | 3 . |
| Rb | 0·08 | 0·04 | 0·08 | 0·44 | 0·30 | 0·4 | 0·23 | 0·20 | 0·15 |
| Sr | 0·00 | 0·16 | 0·16 | 0·24 | 2·12 | 4·4 | 0·23 | 4·41 | 1·01 |
| Y | 0·00 | 0·70 | 0·19 | 0·011 | 0·05 | 8 | 0·55 | 0·24 | 2·88 |
| Nb | 0·00 | 0·05 | 1·50 | 0·01 | 0·00 | 0·4 | 1·5 | 0·00 | 0·39 |
| Ba | 0·04 | 0·04 | 0·14 | 0·09 | 0·24 | 0·1 | 0·14 | 1·08 | 0·61 |
| La | 0·02 | 0·10 | 0·20 | 0·01 | 0·12 | 16 | 0·53 | 0·46 | 0·56 |
| Ce | 0·00 | 0·20 | 0·20 | 0·011 | 0·14 | 16 | 0·56 | 0·36 | 0·87 |
| Nd | 0·00 | 0·60 | 0·20 | 0·013 | 0·07 | 18 | 0·55 | 0·31 | 1·82 |
| Sm | 0·00 | 0·60 | 0·20 | 0·012 | 0·12 | 17 | 0·55 | 0·27 | 2·4 |
| Eu | 0·03 | 0·57 | 0·06 | 0·043 | 0·22 | 3·5 | 0·15 | 1·78 | 2·08 |
| Gd | 0·00 | 0·70 | 0·20 | 0·013 | 0·17 | 7 | 0·55 | 0·30 | 2·5 |
| Dy | 0·00 | 0·70 | 0·20 | 0·013 | 0·03 | 2 | 0·55 | 0·23 | 2·8 |
| Er | 0·00 | 0·70 | 0·20 | 0·014 | 0·15 | 3 | 0·6 | 0·23 | 2·5 |
| Yb | 0·00 | 0·70 | 0·20 | 0·016 | 0·11 | 2 | 0·75 | 0·18 | 1·77 |
| Th | 0·04 | 0·03 | 0·18 | 0·014 | 0·02 | 0·95 | 0·53 | 0·16 | 0·09 |
Ol, olivine; Cpx, clinopyroxene; Fe–Ti Ox, Fe–Ti oxides; Ne, nepheline, Plg, plagioclase, Hbl, hornblende; B, basanite; HW, hawaiite; MG, mugearite; BM, benmoreite. 1, From Villemant et al. (1981) and Lemarchand et al. (1987); 2, from Larsen (1979); 3, from Caroff et al. (1993) except Nb in amphibole (Klein et al., 1997); 4, from Chazot et al. (1996) except Th (Caroff et al., 1993). Values in italics are distribution coefficients extrapolated from those of neighbouring REE.
Present address: UMR 5025 ‘Laboratoire de Géodynamique des Chaînes Alpines’, Université Grenoble 1—Joseph Fourier, Maison des Géosciences, 1381 rue de la Piscine, BP 53, 38041 Grenoble Cedex 9, France.
We thank C. Chauvel for the unpublished isotopic data from Raiatea lavas. Microprobe studies were performed with the help of M. Bohn. Detailed and constructive comments by Dr W. A. Bohrson helped us to improve the manuscript. We also thank Dr R. C. Maury for his judicious suggestions and Drs M. Wilson and R. J. Arculus for their editorial assistance. Field studies were conducted in 1999 with the financial support of DIRCEN, CEA, and BRGM.
REFERENCES
Agemar, T., Wörner, G. & Heumann, A. (
Augé, T., Lerebour, P. & Rançon, J.-P. (
Bardintzeff, J.-M., Bellon, H., Bonin, B., Brousse, R. & McBirney, A. R. (
Binard, N., Hékinian, R., Cheminée, J. L., Searle, R. C. & Stoffers, P. (
Blais, S., Guille, G., Maury, R. C., Guillou, H., Miau, D. & Cotten, J. (
Blais, S., Guille, G., Guillou, H., Chauvel, C., Maury, R. C., Pernet, G. & Cotten, J. (
Bohrson, W. A. & Reid, M. R. (
Bonin, B. & Bardintzeff, J.-M. (
Brandriss, M. E. & Bird, D. K. (
Brousse, R. & Berger, E. T. (
Bryan, W. B., Finger, L. A. & Chayes, F. (
Caroff, M., Maury, R. C., Leterrier, J., Joron, J.-L., Cotton, J. & Guille, G. (
Caroff, M., Maury, R. C., Vidal, P., Guille, G., Dupuy, C., Cotten, J., Guillou, H. & Gillot, P.-Y. (
Caroff, M., Maury, R. C., Guille, G. & Cotten, J. (
Caroff, M., Guillou, H., Lamiaux, M., Maury, M. C., Guille, G. & Cotten, J. (
Chazot, G., Menzies, M. A. & Harte, B. (
Chen, C.-Y. & Frey, F. A. (
Cheng, Q. C., Macdougall, J. D. & Lugmair, G. W. (
Clément, J.-P. & Caroff, M. (
Clément, J.-P., Legendre, C., Caroff, M., Guillou, H., Cotten, J., Bollinger, C. & Guille, G. (
Cotten, J., Le Dez, A., Bau, M., Maury, R. C., Dulski, P., Fourcade, S., Bohn, M. & Brousse, R. (
Dauteuil, O., Blais, S., Miau, D., Caroff, M., Maury, R. C., Dulski, P., Fourcade, S., Bohn, M. & Brousse, R. (
Deer, W. A., Howie, R. A. & Zussman, J. (
Deneufbourg, G. (
Devey, C. W., Albarède, F., Cheminée, J.-L., Michard, A., Mühe, R. & Stoffers, P. (
Dosso, L., Hanan, B. B. & Bougault, H. (
Dostal, J., Dupuy, C. & Liotard, J.-M. (
Duncan, R. A. & Fisk, M. R. (
Duncan, R. A. & McDougall, I. (
Dupuy, C., Barsczus, H. G., Dostal, J., Vidal, P. & Liotard, J-.M. (
Edgar, A. D. (
Feigenson, M. D., Hofmann, A. W. & Spera, F. J. (
Fodor, R. V., Rudek, E. A. & Bauer, G. R. (
Freundt-Malecha, B., Schmincke, H.-U. & Freundt, A. (
Gautier, I., Weis, D., Mennessier, J.-P., Vidal, P., Giret, A. & Loubet, M. (
Ghiorso, M. S. (
Gillis, K. M. & Meyer, P. S. (
Giret, A., Bonin, B. & Léger, J.-M. (
Giret, A., Chotin, P. & Verdier, O. (
Guillou, H., Blais, S., Guille, G., Maury, R. C., Le Dez, A. & Cotten, J. (
Hart, S. R. & Zindler, A. (
Hémond, C., Devey, C. W. & Chauvel, C. (
Hildenbrand, A., Gillot, P.-Y. & Le Roy, I. (
Hoover, S. R. & Fodor, R. V. (
Ielsh, G., Caroff, M., Barsczus, H., Maury, R. C., Guillou, H., Guille, G. & Cotton, J. (
Klein, M., Stosch, H.-G. & Seck, H. A. (
Larsen, L. M. (
Larsen, R. B. & Brooks, C. K. (
Leake, B. E., Woolley, A. R., Arps, C. E. S., et al. (
Le Bas, M. J. & Streckeisen, A. L. (
Le Dez, A., Maury, R. C., Guillou, H., Cotten, J. & Brousse, R. (
Le Dez, A., Maury, R. C., Guillou, H., Cotten, J., Blais, S. & Guille, G. (
Legendre, C., Maury, R. C., Guillou, H., Cotten, J., Caroff, M., Blais, S. & Guille, G. (
Lemarchand, F., Villemant, B. & Calas, G. (
Le Roy, I. (
Marcelot, G., Bardintzeff, J.-M., Maury, R. C. & Rançon, J.-P. (
Mathison, C. I. (
McBirney, A. R. & Aoki, K. J. (
Metzner, C. & Grimmeisen, W. (
Middlemost, E. A. K. (
Mitchell, A. A., Naicker, S. B., Marsh, J. S. & Dunlevey, J. N. (
Nekvasil, H., Simon, A. & Lindsley, D. H. (
Nitecki-Novotny, S. (
Phinney, W. C. (
Rançon, J.-P., Lerebour, P. & Augé, T. (
Roeder, P. L. & Emslie, R. F. (
Schandl, E. S., Gorton, M. P. & Wicks, F. J. (
Schirnick, C., Van den Bogaard, P. & Schmincke, H. U. (
Sisson, T. W. & Grove, T. L. (
Sørensen, H. (
Späth, A., Le Roex, A. P. & Duncan, A. R. (
Staudigel, H. & Schmincke, H. U. (
Streckeisen, A. L. (
Streckeisen, A. L. (
Sun, S. S. & McDonough, W. F. (
Tepley, F. J., III, Davidson, J. P., Tilling, R. I. & Arth, J. G. (
Thompson, G. M., Smith, I. E. M. & Malpas, J. G. (
Tribuzio, R., Tiepolo, M. & Thirlwall, M. F. (
Villemant, B., Jaffrezic, H., Joron, J.-L & Treuil, M. (
Wager, L. R., Brown, G. M. & Wadsworth, W. J. (
Walker, G. P. L. (
Wedepohl, K. H. (
White, W. M. (
White, W. M. & Duncan, R. A. (
White, W. M. & Hofmann, A. W. (
Wright, T. L. & Doherty, P. C. (


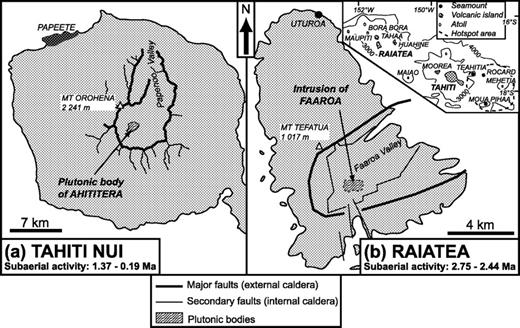
![Location of the studied samples. Four samples were collected during previous field trips [the Ahititera monzonite 37H: Bardintzeff et al. (1988) and the Faaroa gabbro RI-28, and theralites RI-85 and RI-86: Blais et al. (1997)]. Geological sketch maps of (a) the Ahititera plutonic body (Tahiti Nui) and its surroundings and (b) the Faaroa depression (Raiatea). Circled symbols are Raiatea samples.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/petrology/46/11/10.1093/petrology/egi055/2/m_egi055f2.jpeg?Expires=1716541948&Signature=CCtWmtE5MfLRdi2Bo8aTCV-zK3QCQJgSy~Qd5ZJ5J0Dy7fuZkyN39ASciYjrFuUvjsEII2ATvr7JDJF~Uh5vGb3xXAs-2M0QJODYp45395d-rWhomr-iqh-~TYSfPW9bdB7acdxKPxWxy-nLFRhERVy2jLs~aUAk69aXfvDa~DQRJ6W3hL-F2H8c-sKhoIhw-bsp60TBvvL0bjS4mda9Teo65mmjTM4CNgeb2d9WGaACvlC0Ne3LC1TyQcTMeehFUC6sGAe9IVbyB9-jP7cWXGlaYLaUvv7IiTmSag9HlIP0KrjDexiJ5cqe~TAjFb~fgvOQQd8Pd24fBJslf7ynMA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
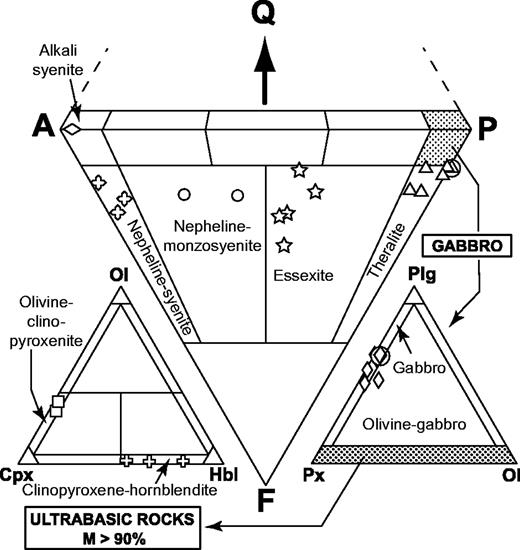
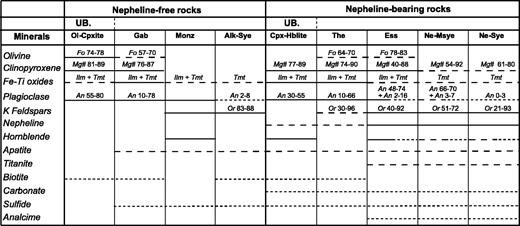
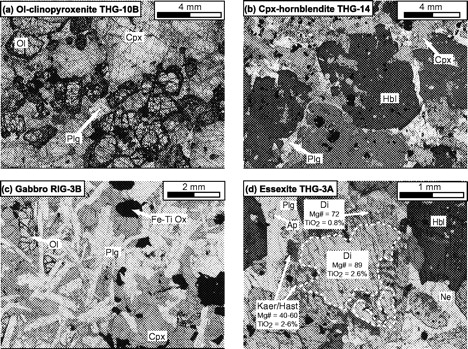
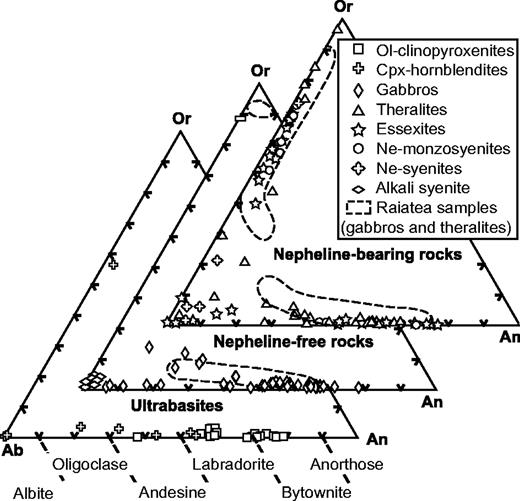
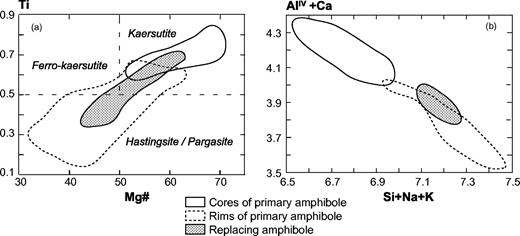
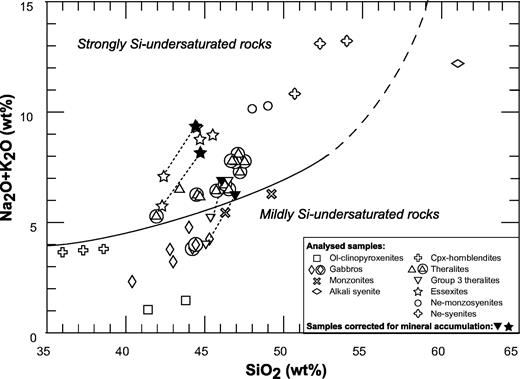
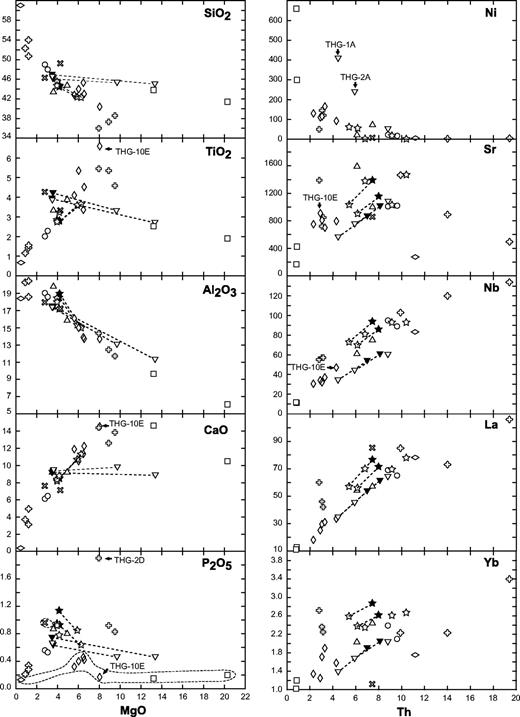
![C1 chondrite-normalized REE patterns of representative samples from Ahititera and Faaroa [normalization values from Sun & McDonough (1989)]. Circled symbols are Raiatea samples.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/petrology/46/11/10.1093/petrology/egi055/2/m_egi055f10.jpeg?Expires=1716541948&Signature=OutRFEzwk-AZoZI09572IKv6hRVekFZhkWv2jkns4ibNKrSudL1QOVxAcB9RrLMwsEAwrj0exW9PMMSSeqi8QYZbFvNUEfR-P9RQK-rI-JWnCEOZZLLqLPUYxl6vf3QbiOx4esUX6QDb5vja5ZmyjFN49ayEFhdtwznNrMdsa-A0678-v1Fy~SLTD8jz7EPqePTIlDo6TTgMf7AFfwZFB1LhN-VQFxEtDXHC8wdEmY1PEaR-gnERft61eZP5zHEXieo9J5e~LEVVv2J4S~7DqwrxxeBlDhWfwU~ujtWF3Zh4PbtIgIgW2eYdF1vPmKYvonvLRVjxwa4ItwzfdimhmA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
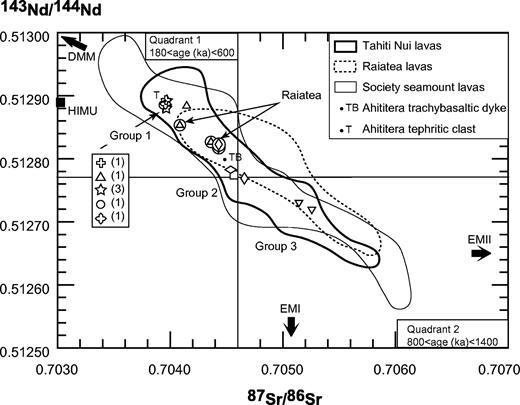
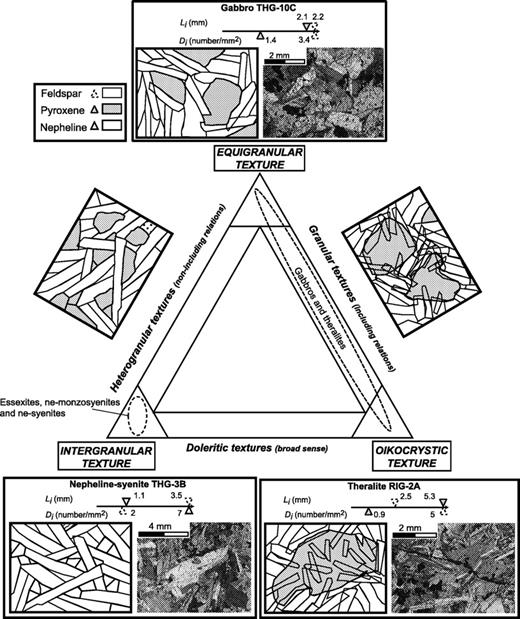
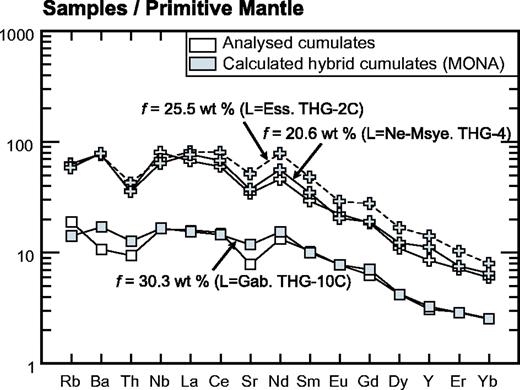
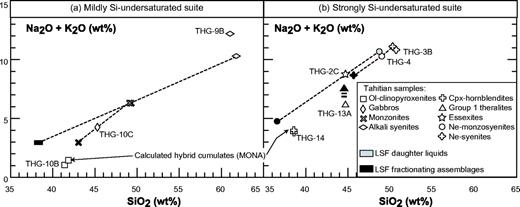
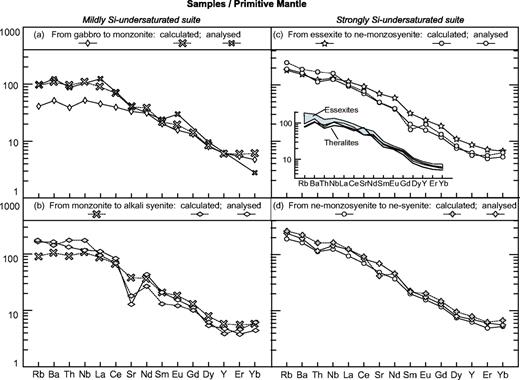
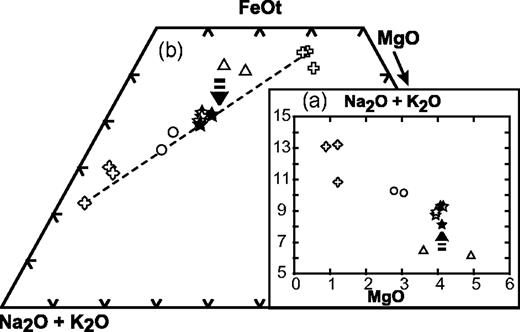
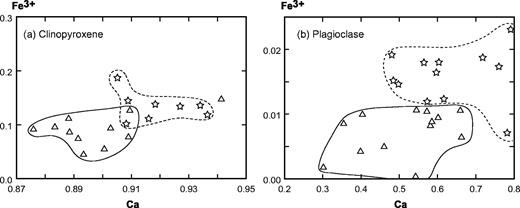
![Comparison between gabbros and theralites from the Ahititera pluton. Two of the Group 3 theralites are corrected to take account of mineral accumulation. (a) C1 chondrite-normalized REE patterns [normalization values from Sun & McDonough (1989)]. (b) Plot of Th/Nb vs La/Yb for Ahititera mafic samples.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/petrology/46/11/10.1093/petrology/egi055/2/m_egi055f18.jpeg?Expires=1716541948&Signature=I5Hn6xVRMfEL1zpksBoC5IMLP~98ZIlA8GwwgLzo9q~eUPRK2FqcA-VM8~3DQyotVWCk~vXIAFLo7eQGAmj4rg2kbkEMz9cUsspia9Rc9AvgqJLU5zkFOT69ObOCWl3Y6HQD9WHPQsFQHEy4o7n9QWAwclT5X-OiS2paEqOgPbbTQNLYHjMQda9bEdtiW2h7lsHt9OpymAvPU2AdUlZvopSxqUcB3mNBzUQKQ3Lnn6pn1U0KsG7ubm8m8iH9Yp5IfNFGcBBJ~6OjWnAhxY7jD4FVcLu74mZlcASNXECe73nJoGVnedliQXZhD4W6HR3fDqpslzA8xml7fzYszkGz2w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Comparison between REE patterns of strongly and mildly Si-undersaturated mafic rocks from the Society Islands [normalization values from Sun & McDonough (1989)]. (a) Fields of mafic coarse-grained rocks (theralites and gabbros) from Tahiti Nui and Raiatea plutons. Theralites THG-1A and -2A from Tahiti Nui are corrected to take account of mineral accumulation. (b) REE patterns of mafic lavas (3 wt % < MgO < 11 wt %: basanites and basalts) from Moua Pihaa, Teahitia, and Yves Rocard seamounts (Dupuy et al., 1989; Hémond et al., 1994). Inset: fields of basanites (n = 22) and basalts (n = 39) from Tahiti Nui and Raiatea islands (for Raiatea: unpublished data, Blais et al., 1997; for Tahiti Nui: unpublished data, Dostal et al., 1982; Hémond et al., 1994; Le Roy, 1994; Clément et al., 2002).](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/petrology/46/11/10.1093/petrology/egi055/2/m_egi055f19.jpeg?Expires=1716541948&Signature=mC0tzHgi0HIpN3KfHpLhKV3uIPAr6PxbJFTNLVbuPSfTvYm8x1OsfDU860P3RSYOeTTZto7N-iu8XDVRH6gpNBTnRCXCJPf36gNGqJ8LO8zFzLHPLKag6fckMABp5NI4DB1M-4HkWjHJIHkjO27Y-7FxadjU7lALwfU1u~MhplRE2EgY2781lRwmqExH~5vugS7AAAFvK1Es2aWgXfChXOSPMK3ajgiy3XLKQDsO~o21~ufAORIRsuL9Yle1dMKbf4pxYcIJvqLGm0o9wIbmOR-hcomJfMpauyJcZN-ylZdNh5fEb3gNh-wBcMVk41Mc3l6qTJqPQnTjqxzSBkPJiw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)