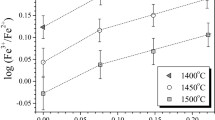
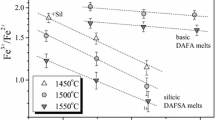
Abstract
The influence of melt composition and structure on the oxygen isotope fractionation was studied for the multicomponent (SiO2 ± TiO2 + Al2O3 ± Fe2O3 + MgO ± CaO) system at 1500°C and 1 atm. The experiments show that significant oxygen isotope effects can be observed in silicate melts even at such high temperature. It is shown that the ability of silicate melt to concentrate 18O isotope is mainly determined by its structure. In particular, an increase of the NBO/T ratio in the experimental glasses from 0.11 to 1.34 is accompanied by a systematic change of oxygen isotope difference between melt and internal standard by values from–0.85 to +1.29‰. The obtained data are described by the model based on mass-balance equations and the inferred existence of O0, O–, and O2– (bridging, non-bridging, and free oxygen) ions in the melts. An application of the model requires the intra-structure isotope fractionation between bridging and non-bridging oxygens. Calculations show that the intra-structure isotope fractionation in our experiments is equal to 4.2 ± 1.0‰. To describe the obtained oxygen isotope effects at the melts relatively to temperature and fraction of non-bridging oxygen a general equation was proposed.
Similar content being viewed by others
References
Anfilogov, V.N., Bykov, V.N., and Osipov, A.A., Silikatnye rasplavy (Silicate Melts), Moscow: Nauka, 2005.
Appora, I., Eiler, J.M., Matthews, A., and Stolper, E.M., Experimental determination of oxygen isotope fractionations between CO2 vapor and soda–melilite melt, Geochim. Cosmochim. Acta, 2003, vol. 67, pp. 459–471.
Ariskin, A.A. and Polyakov, V.B., Simulation of molecular mass distributions and evaluation of O2–concentrations in polymerized silicate melts, Geochem. Int., 2008, vol. 46, no. 5, pp. 429–447.
Bigeleisen, J. and Mayer, M.G., Calculation of equilibrium constants for isotope exchange reactions, J. Chem. Phys., 1947, vol. 15, pp. 261–267.
Borisov, A., Loop technique: dynamic of metal/melt equilibration, Mineral. Petrol., 2001, vol. 71, pp. 87–94.
Borisov, A.A. and Dubinina, E.O., Effect of network-forming cations on the oxygen isotope fractionation between silicate melts: experimental study at 1400–1570oC, Petrology, 2014, vol. 22, no. 4, pp. 359–380.
Borisov, A., Behrens, H., and Holtz, F., The effect of titanium and phosphorus on ferric/ferrous ratio in silicate melts: an experimental study, Contrib. Mineral. Petrol., 2013, vol. 166, pp. 1577–1591.
Borisov, A., Behrens, H., and Holtz, F., Effects of melt composition on Fe3+/Fe2+ in silicate melts: a step to model ferric/ferrous ratio in multicomponent systems, Contrib. Mineral. Petrol., 2015, vol. 169.
Borisov, A., Behrens, H., and Holtz, F., Effects of strong network modifiers on Fe3+/Fe2+ in silicate melts: an experimental study, Contrib. Mineral. Petrol., 2017, vol. 172.
Bucholz, C.E., Jagoutz, O., Van Tongeren, J.A., et al., Oxygen isotope trajectories of crystallizing melts: insights from modeling and the plutonic record, Geochim. Cosmochim. Acta, 2017, vol. 207, pp. 154–184.
Canil, D. and Muehlenbachs, K., Oxygen diffusion in an Fe-rich basalt melt, Geochim. Cosmochim. Acta, 1990, vol. 54, pp. 2947–2951.
Chacko, T., Cole, D.R., and Horita, J., Equilibrium oxygen, hydrogen and carbon isotope fractionation factors applicable to geological systems, Rev. Mineral., 2001, vol. 43, pp. 1–81.
Clayton, R.N. and Kieffer, S.W., Oxygen isotope thermometer calibrations, Stable Isotope Geochemistry: A Tribute to Samuel Epstein, Taylor, H.P., Jr, O’Neil, J.R., Kaplan, I.R., Eds., Geochem. Soc. Spec. Publ., 1991, no. 3, pp. 3–10.
Coplen, T.B., Reporting of stable hydrogen, carbon, and oxygen isotopic abundances, Pure Appl. Chem., 1994, vol. 66, pp. 273–276.
Dalou, C., Le Losq, C., and Mysen, B.O., In situ study of the fractionation of hydrogen isotopes between aluminosilicate melts and coexisting aqueous fluids at high pressure and high temperature: implications for the dD in magmatic processes, Earth Planet. Sci. Lett., 2015, vol. 426, pp. 158–166.
Duffy, J.A., A review of optical basicity and its applications to oxidic systems, Geochim. Cosmochim. Acta, 1993, vol. 57, pp. 3961–3970.
Dunn, T., Oxygen diffusion in three silicate melts along the join diopside–anorthite, Geochim. Cosmochim. Acta, 1982, vol. 46, pp. 2293–2299.
Eiler, J.M., Oxygen isotope variations of basaltic lavas and upper mantle rocks, Stable Isotope Geochemistry, Valley, J.W. and Cole, D.R., Eds., Rev. Mineral, 2001, vol. 43, pp. 319–364.
Esin, O.A. Polymineral model of melted silicates, in Solutions. Melts: Results of Science and Technique, tMoscow: VINITI, 1975, vol. 2, pp. 76–107.
Fincham, C.J.B. and Richardson, F.D., The behavior of sulfur in silicate and aluminate melts, Proc. R. Soc. London: Ser. A, 1954, vol. 223.
Garlick, G.D., Oxygen isotope fractionation in magmatic rocks, Earth Planet. Sci. Lett., 1966, vol. 1, pp. 361–368.
Hao, X. and Wang, X., A new sulfide capacity model for CaO–Al2O3–SiO2–MgO slags based on corrected optical basicity, Steel Res. Intern., 2017, vol. 87, pp. 359–363.
Hess, P.C., Polymer model of silicate melts, Geochim. Cosmochim. Acta, 1971, vol. 36, pp. 289–306.
Kroopnick, P. and Craig, H., Atmospheric oxygen: isotopic composition and solubility fractionation, Science, 1972, vol. 175, pp. 54–55.
Kyser, T.K., Lesher, C.E., and Walker, D., The effects of liquid immiscibility and thermal diffusion on oxygen isotopes in silicate liquids, Contrib. Mineral. Petrol., 1998, vol. 133, pp. 373–381.
Labidi, J., Shahar, A., Le Losq, C., et al. Experimentally determined sulfur isotope fractionation between metal and silicate and implications for planetary differentiation, Geochim. Cosmochim. Acta, 2016, vol. 175, pp. 181–194.
Lesher, C.E., Self-diffusion in silicate melts: theory, observations and applications to magmatic systems, Rev. Mineral. Geochem., 2010, vol. 72, pp. 269–309.
Lester, G.W., Kyser, T.K., and Clark, A.H., Oxygen isotope partitioning between immiscible silicate melts with H2O, P and S, Geochim. Cosmochim. Acta, 2013, vol. 109, pp. 306–311.
Le Losq, C., Mysen, B.O., and Cody, G.D., Intramolecular fractionation of hydrogen isotopes in silicate quenched melts, Geochem. Persp. Lett., 2016, vol. 2, pp. 87–94.
Masson, C.R., Ionic equilibria in liquid silicates, J. Am. Ceram. Soc., 1968, vol. 51, pp. 134–143.
Masson, C.R., Smith, I.B., and Whiteway, S.G., Activities and ionic distributions in liquid silicates: application of polymer theory, Can. J. Chem., 1970, vol. 48, pp. 1456–1464.
Matthews, A., Palin, J.M., Epstein, S., and Stolper, E.M., Experimental study of 18O/16O partitioning between crystalline albite, albitic glass and CO2 gas, Geochim. Cosmochim. Acta, 1994, vol. 58, pp. 5255–5266.
Mills, K.C., The influence of structure on the physicochemical properties of slags, ISIJ Int., 1993, vol. 33, pp. 148–155.
Moretti, R., Polymerisation, basicity, oxidation state and their role in ionic modelling of silicate melts, Ann. Geophys., 2005, vol. 48, pp. 583–607.
Muehlenbachs, K. and Kushiro, I., Oxygen isotope exchange and equilibrium of silicates with CO2 and O2, Carnegie Inst. Wash. Yearbook, 1974, vol. 73, pp. 232–236.
Mungall, J.E., Empirical models relating viscosity and tracer diffusion in magmatic silicate melts, Geochim. Cosmochim. Acta, 2002, vol. 66, pp. 125–143.
Mysen, B.O., Aluminosilicate melts: structure, composition and temperature, Contrib. Mineral. Petrol., 1997, vol. 127, pp. 104–118.
Mysen, B.O., Hydrogen isotope fractionation between coexisting hydrous melt and silicate-saturated aqueous fluid: an experimental study in situ at high pressure and temperature, Am. Mineral., 2013, vol. 98, pp. 376–386.
Mysen, B.O. and Richet, P., Silicate Glasses and Melts: Properties and Structure, Amsterdam: Elsevier, 2005.
Mysen, B.O. and Fogel, M.L., Nitrogen and hydrogen isotope compositions and solubility in silicate melts in equilibrium with reduced (N + H)-bearing fluids at high pressure and temperature: effects of melt structure, Am. Mineral., 2010, vol. 95, pp. 987–999.
Mysen, B.O., Virgo, D., and Seifert, F.A., Relationships between properties and structure of aluminosilicate melts, Am. Mineral., 1985, vol. 70, pp. 88–105.
Palin, J.M., Epstein, S., and Stolper, E., Oxygen isotope partitioning between rhyolitic glass/melt and CO2: an experimental study at 550–950°C and 1 bar, Geochim. Cosmochim. Acta, 1996, vol. 60, pp. 1963–1973.
Qin, T., Wu, F., Wu, Z., and Huang, F., First principles calculations of equilibrium fractionation of O and Si isotopes in quartz, albite, anorthite and zircon, Contrib. Mineral. Petrol., 2016, vol. 171, p. 91.
Schuessler, J.A., Botcharnikov, R.E., Behrens, H., et al., Oxidation state of iron in hydrous phono-tephritic melts, Am. Mineral., 2008, vol. 93, pp. 1493–1504.
Sharp, Z.D., A laser-based microanalytical method for the in situ determination of oxygen isotope ratios in silicates and oxides, Geochim. Cosmochim. Acta, 1990, vol. 54, pp. 1353–1357.
Stolper, E. and Epstein, S., An experimental study of oxygen isotope partitioning between silica glass and CO2 vapor, Stable Isotope Geochemistry: A Tribute to Samuel Epstein, Taylor, H.P., Jr, O’Neil, J.R, and Kaplan, I.R., Eds., Geochem. Soc. Spec. Publ., 1991, vol. 3, pp. 35–51.
Toop, G.W. and Samis, C.S., Activities of ions in silicate melts, Transactions of the Metallurgical Society of America, 1962, vol. 224, pp. 878–887.
Valley, J.W., Kitchen, N., Kohn, M.J., et al., UWG-2, a garnet standard for oxygen isotope ratios: strategies for high precision and accuracy with laser heating, Geochim. Cosmochim. Acta, 1995, vol. 59, pp. 5223–5231.
Wang, Y., Cody, S.X., Foustoukos, D., et al., Very large differences in intramolecular D–H partitioning in hydrated silicate melts synthesized at upper mantle pressures and temperatures, Am. Mineral., 2015, vol. 100, pp. 1182–1189.
Wendlandt, R.F., Oxygen diffusion in basalt and andesite melts: experimental results and discussion of chemical versus tracer diffusion, Contrib. Mineral. Petrol., 1991, vol. 108, pp. 463–471.
Zhao, Z.F. and Zheng, Y.F., Calculation of oxygen isotope fractionation in magmatic rocks, Chem. Geol., 2003, vol. 193, pp. 59–80.
Zheng, Y.F., Calculation of oxygen isotope fractionation in metal oxides, Geochim. Cosmochim. Acta, 1991, vol. 55, pp. 2299–2307.
Zheng, Y.F., Calculation of oxygen isotope fractionation in anhydrous silicate minerals, Geochim. Cosmochim. Acta, 1993, vol. 57, pp. 1079–1091.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © E.O. Dubinina, A.A. Borisov, 2018, published in Petrologiya, 2018, Vol. 26, No. 4, pp. 426–441.
Rights and permissions
About this article
Cite this article
Dubinina, E.O., Borisov, A.A. Structure and Composition Effects on the Oxygen Isotope Fractionation in Silicate Melts. Petrology 26, 414–427 (2018). https://doi.org/10.1134/S0869591118040021
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0869591118040021