An Operational Framework for the Advancement of a Molecule-to-Biosphere Stoichiometry Theory
- 1Department of Ecology and Environmental Science, Umeå University, Umeå, Sweden
- 2Alfred-Wegener-Institut Helmholtz-Zentrum für Polar- und Meeresforschung, Biologische Anstalt Helgoland, Helgoland, Germany
- 3Ecology and Biodiversity, Department Biology, Vrije Universiteit Brussel, Brussels, Belgium
- 4Departments of Aquatic and Terrestrial Ecology, Netherlands Institute of Ecology (NIOO-KNAW), Wageningen, Netherlands
Biological stoichiometry is an approach that focuses on the balance of elements in biological interactions. It is a theory that has the potential to causally link material processes at all biological levels—from molecules to the biosphere. But the lack of a coherent operational framework has so far restricted progress in this direction. Here, we provide a framework to help infer how a stoichiometric imbalance observed at one level impacts all other biological levels. Our framework enables us to highlight the areas of the theory in need of completion, development and integration at all biological levels. Our hope is that this framework will contribute to the building of a more predictive theory of elemental transfers within the biosphere, and thus, to a better understanding of human-induced perturbations to the global biogeochemical cycles.
Introduction
Ecological stoichiometry studies the balance of chemical elements in ecological interactions (Sterner and Elser, 2002). Most hypotheses in ecology are born from the clever integration of previously unrelated assumptions and observations (Pickett et al., 2007d). Ecological stoichiometry is a great illustration of this principle since it came as a eureka combination of two observations: the demonstration that zooplankton can increase the growth rate of phytoplankton through the recycling of limiting nutrients (Sterner, 1986) and the shift in phytoplankton limitation between phosphorus (P) and nitrogen (N) that sometimes results from manipulations of the zooplankton community species composition (Elser et al., 1988). The result was a model of how zooplankton N and P composition could drive phytoplankton limitation through differential recycling of N and P (Sterner, 1990). The hypothesis was then called the Consumer-Driven Nutrient Recycling Hypothesis (Sterner et al., 1992). Hypotheses grow into fully-fledged theories through further integration of new observations, concepts, hypotheses and models, thus increasing their contribution to the understanding of the phenomena they are meant to explain (Pickett et al., 2007c; Marquet et al., 2014). Accordingly, the Consumer-Driven Nutrient Recycling hypothesis grew up into the field of ecological stoichiometry by incorporating more hypotheses, such as the growth rate and light:nutrient hypotheses (Sterner et al., 1997; Elser et al., 2000c). The depth and breadth of ecological stoichiometry then expanded through the integration of empirical observations and experimental results from increasingly diverse habitats, ecosystems and trophic interactions (Sardans et al., 2012). One cannot help but be struck by the vitality of the theory, expressed in a large number of mechanistic hypotheses and testable predictions, as well as in the wide scope of biological phenomena it now encompasses: from freshwater plankton interactions to, for example, the evolution of terrestrial plant genomes (Acquisti et al., 2009), the growth of cancerous tumors (Elser et al., 2003) or the macroevolution of life during the early Cambrian period (Elser et al., 2006). Thus, the theory shows the greatest potential in the midst of many contemporary ecological theories to unify ecology across all biological levels, from molecules to the biosphere (Sterner and Elser, 2002; Elser, 2006; Hessen et al., 2013). Some even argue, with good reason, that the theory should now be called “Biological Stoichiometry,” since it no longer restricts itself to the study of ecological patterns (Elser et al., 2000c).
But completeness—breadth of scope and diversity of components—is not the only axis along which a theory can grow (Sterner and Schulz, 1998). Two other axes are the development of each component toward more realism and applicability; and integration, the connection among components toward better articulation (Pickett et al., 2007c). The ultimate objective of a theory being to generate understanding, it must also be judged by the advancement it provides to our comprehension of natural patterns (Pickett et al., 2007a; Marquet et al., 2014). Only when organized into a logical framework that assembles its components into an explicit structure, can a theory correctly predict or explain occurring natural patterns (Pickett et al., 2007b). Natural patterns are generally the result of complex multi-scale hierarchical processes (O'Neill, 1986), and so, most of the fundamental and urgent questions in ecology nowadays are multi-scale and integrative (Irschick et al., 2013; Sutherland et al., 2013). The logical framework of an ecological theory should thus typically be nested and hierarchical as well as integrated, both in terms of its internal components and with other theories.
In the case of biological stoichiometry, foundations for such a logical framework have already been laid, notably in the canon book by Sterner and Elser (2002) and more recently in review articles (Hall, 2009; Sardans et al., 2012; Hessen et al., 2013). Despite offering a panorama of the insights gained from applying the stoichiometric approach to processes spanning from the physiological to the ecosystem level, those reviews do not provide the kind of framework that is needed to help the theory progress along the three axes of completeness, development and integration. Not enough efforts have been made to integrate and organize the various stoichiometric breakthroughs into one coherent framework. Moreover, links between mechanisms at the lower and higher ends of the biological organization levels are often inferred but seldom fully explicated (Schade et al., 2005).
Here, we offer a methodological framework that describes the processes that need to be investigated at each biological level in order to characterize the repercussions of a stoichiometric imbalance at one level over all the other levels. This framework is operational rather than descriptive. It explains “how to” derive consequences of stoichiometric imbalances rather than “what” will be those consequences. As such, it is adaptable to all biological systems and open to further development. Armed with this framework, we surveyed the literature, in order to assess the current understanding of stoichiometric patterns and processes at each biological level (see our framework on Figure 1). We focus particularly on the degree of completeness of current understanding (i.e., whether are there any important considerations missing or not), the degree of development (how reliable are the proposed concepts and hypotheses?) and the degree of integration (how are other theories, approaches and knowledge considered and included?). We selected readings from the stoichiometry literature, from seminal publications by Liebig and Playfair (1840) and Redfield (1934) to the most recent literature (>900 articles read). Articles were first selected using generic search engines (Web of Science, Scopus) and generic keywords (biological OR ecological AND stoichiometr*). Articles were then more selectively pursued according to their relevance to the component of the framework evaluated and the elements that we assessed as in need of completion, development or integration. Our evaluation of each framework component is discussed below in separate sections. We have also summarized the elements that we deemed in need of enhancement according to the three axes of completeness, development and integration in Tables 1, 2.
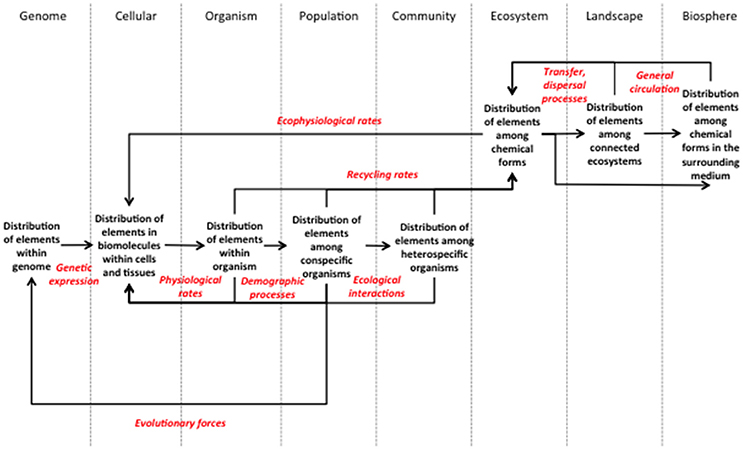
Figure 1. Framework for a molecule-to-biosphere stoichiometry theory. This framework presents explicitly the biological levels and processes that need to be investigated in order to characterize the repercussions of a stoichiometric imbalance at one level over all the other levels. Each biological level should be described by the patterns of elemental distribution within it. As the framework shows, the processes investigated should go beyond the fluxes and rates of elements between components that are classically considered in stoichiometric studies. Any process affecting the structure of a biological level should be considered if the changes provoked relate to changes in the distribution of elements. Numerous examples are discussed within the text.
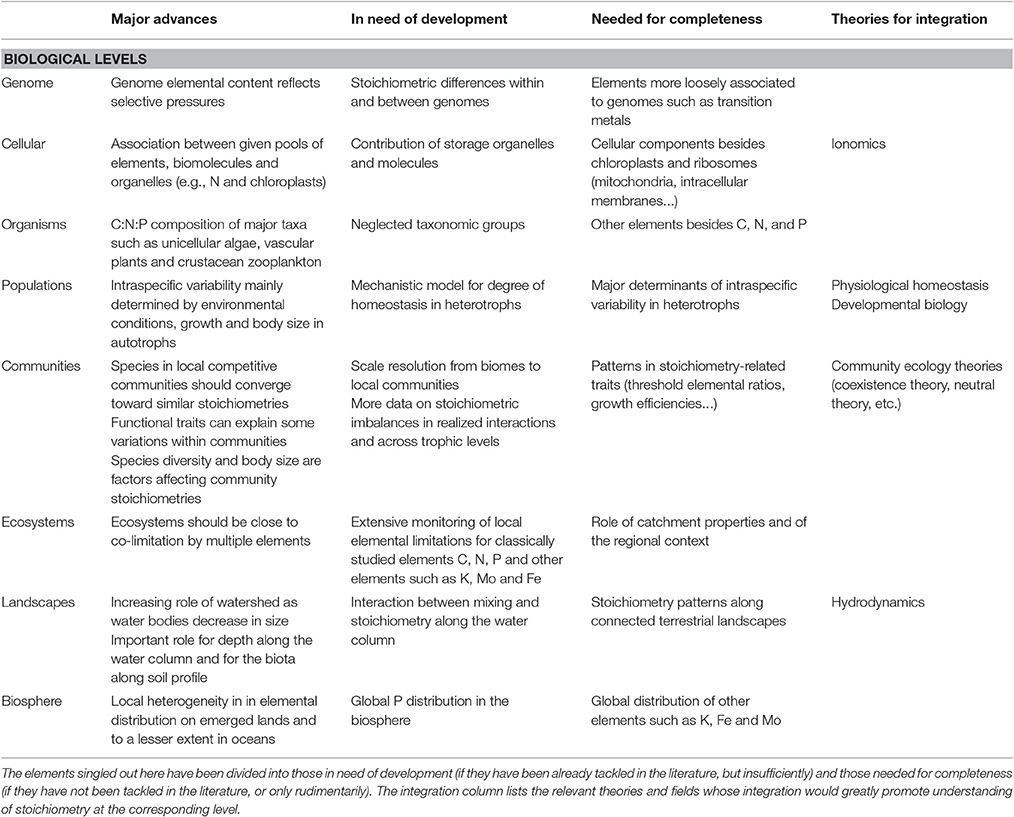
Table 1. Brief summary of the major advances at each biological level of our framework for a molecule-to biosphere stoichiometry theory and of the elements of understanding identified as in need of improvement.
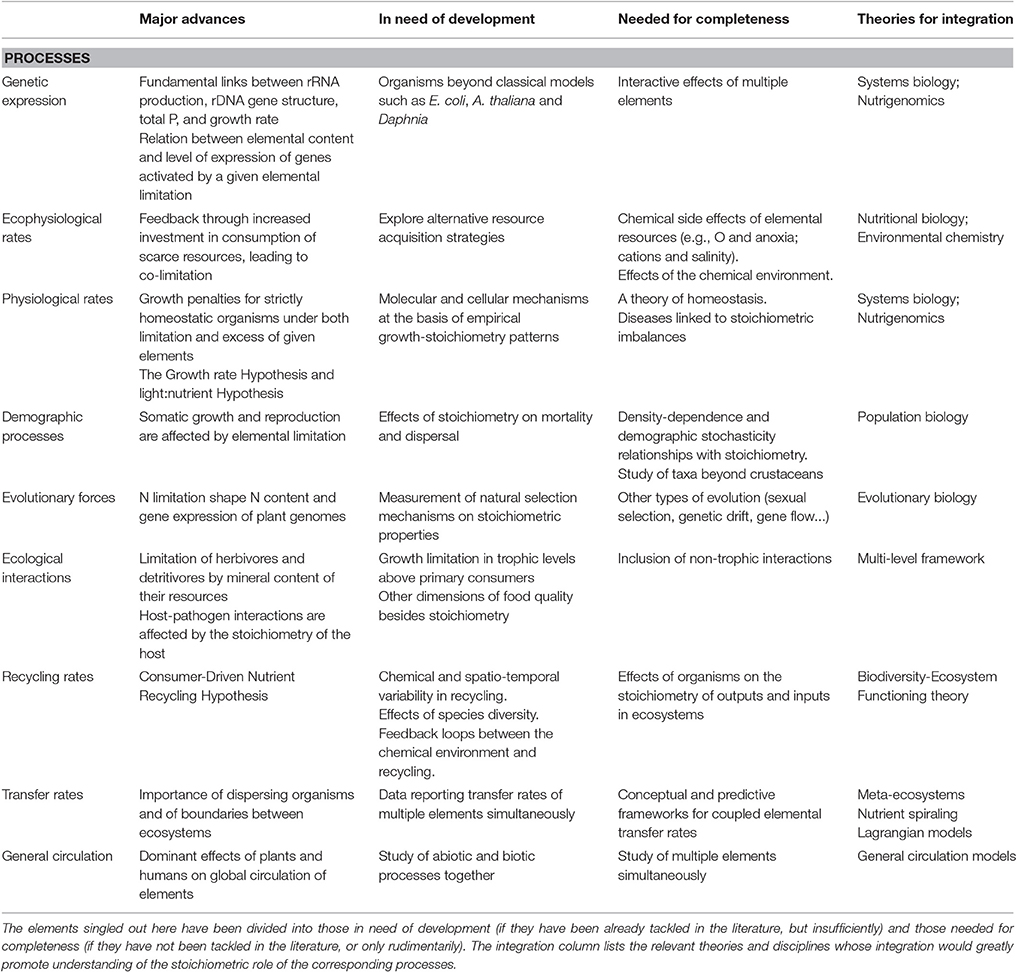
Table 2. Brief summary of the major advances at each biological level of our framework for a molecule-to biosphere stoichiometry theory and of the elements of understanding identified as in need of improvement for each process of our framework for a molecule-to biosphere stoichiometry theory.
Framework Definition
Our framework is organized along a rendition of the classical biological levels, defined by their stoichiometry, the distribution of elements within them, and connected by the processes that mediate the repercussions of changes in the stoichiometry of one level upon another (Figure 1). We are aware that there is no universally agreed-upon description of hierarchical levels in biology, arguably because such a description depends to a large extent on the specificities of the system studied (Allen and Hoekstra, 1993). So, we open the possibility for adapting the choice and definition of levels to the specificities of the systems to which the framework is applied. Also, we acknowledge that the highest conventional biological levels can be defined at any spatial and temporal scale (Allen and Hoekstra, 1990). Ecosystems for example can be as small as a drop of water or as incommensurable as an ocean (O'Neill, 1986). So the landscape and biosphere levels in our framework should not be understood according to the common-sense definition of the terms. We define a landscape as a set of connected ecosystems, themselves operational units defined according to the aims of the investigators, be it a moss patch, a water pool or a continental forest. The biosphere is the surrounding medium that circulates unbounded between the ecosystem units. Populations are the sum of individuals from the same species (or any other relevant taxonomic unit) contained in one ecosystem unit that can reproduce or exchange genetic information at the timescale considered; community is the ensemble of populations present in a unit ecosystem. Levels from the organism and below are scale independent. We also include tissues as part of the cellular level to the extent that the cells that compose them answer uniformly to the factors affecting their stoichiometry.
The other elements of the framework are the processes that relate changes in the elemental distribution of a given level to the stoichiometric properties of the other levels (Figure 1). They are the medium by which imbalances in the stoichiometry of one level reverberate throughout all biological levels. In practical terms, they are the processes whose rates have to be measured by investigators interested in the multi-scale effects of stoichiometry. The framework reveals that the cellular, organism and ecosystem levels are essential nodes that cannot be bypassed when connecting high and low levels (Figure 1). Obviously, regional processes may affect individual organisms only if they influence the local ecosystem that embeds them. Within the local ecosystem, changes in the distribution of elements among chemical forms may impact organisms only if they affect at least one ecophysiological rate, altering the distribution of elements within cells. This highlights the central role of metabolism and ecosystem processes in connecting the biotic and abiotic cycles of elements. Ecophysiological rates and evolutionary forces are the only processes that enable the highest levels to reach the lowest levels (genomic and cellular). They are also the processes that allow organisms to adapt to changes in stoichiometry at larger scales. Biotic adaptation can then lead to further larger-scale changes through recycling processes, thus closing the feedback loop of stoichiometry from molecules to the biosphere.
Biological Levels
Stoichiometric studies can be found for all levels, some of them having acquired a foundational character in ecology, often solidifying into paradigms (e.g., Liebig and Playfair, 1840; Redfield, 1958; Tilman, 1982). The emergence of the biological stoichiometry approach made use of these paradigms, sometimes unaltered, sometimes developing them further, and sometimes challenging them (e.g., for the Redfield ratio paradigm, Cleveland and Liptzin, 2007; Sterner et al., 2008; Flynn, 2010; Loladze and Elser, 2011; Sardans et al., 2012). New paradigms have also been developed for various biological levels, and integrations with other ecological theories attempted. In this section, we briefly discuss the contribution of stoichiometric approaches to our understanding of the elemental distribution within each biological level, as well as the areas still in need of improvement, starting from genomes, the lowest level, to the biosphere.
Genome
Nucleic acids are known to be N-rich and, singularly, P-rich molecules (Sterner and Elser, 2002). But only recently has the new field of stoichiogenomics started working on a comparison between the elemental compositions of the genomes of various organisms (Elser et al., 2011). Intriguing differences in N content between wild plants and domesticated crops were revealed (Acquisti et al., 2009). As one Guanine-Cytosine (GC) pair contains 8 N atoms, one more than an Adenine-Thymine (AT) pair, correlations between the GC and N contents of genomes has been postulated (McEwan et al., 1998) but hotly debated (Bragg and Hyder, 2004). Correlation between genome size and P content is also controversial (Hessen et al., 2010b; Vieira-Silva et al., 2010). There is another more subtle way for stoichiometric constraints to imprint on the genome of organisms. The N contents of codons and the amino acids they encode are positively correlated, resulting in a strong relationship between the nitrogen:carbon (N:C) ratio of genomes and proteomes across prokaryotic species (Bragg and Hyder, 2004). Hence, organisms under selection for lower N in their proteins should also see their genome N content decrease, although many other genomic processes may obscure the picture (Gunther et al., 2013). Regarding P, it is the causal association between rDNA structure, rRNA expression, cellular P content and growth rate that is likely to affect the structure of genomes (Weider et al., 2005). Despite these notable breakthroughs, the field of stoichiogenomics is at its early stages. Still lacking is information on potential differences in the stoichiometry of various types of sequences (eu- and heterochomatin, introns and exons, transposons, etc.), of distinct physical structures of genomes (chromosomes, telomeres, centromeres, etc.), as well as differences between the genomes of different organelles (e.g., chloroplasts and mitochondria), cell types (e.g., immune cells, or syncitia) and species (Table 1). Other elements beside C, N, and P are worth assessing too. For example, a rather old study by Kearns and Sigee (1979) suggests that some transition metals in cells, like nickel and copper, are mainly associated with chromosomes in dinoflagellates. Thus, differences in genome size among dinoflagellates may result in differences in transition metal contents.
Cellular
Genomes represent only a small fraction of a cell's biomass. Major pools of elements in cells are proteins and nucleotides for N, RNA and phospholipids for P, and carbohydrates and lipids for C (Sterner and Elser, 2002). Hence, the stoichiometry of cells is to all intents and purposes the result of the relative investment of the cell in those few dominant macromolecules (Vrede et al., 2004). This fundamental understanding allows us to predict the stoichiometry of cells in many cases. For example, actively photosynthesizing leaves should show high N:C ratios, because of their heavy investment in RubisCo and other photosynthesis proteins (Field and Mooney, 1986; Evans, 1989). The Growth Rate Hypothesis (Elser et al., 1996) states that fast growing cells should have high contents of ribosomal RNA, and thus also of P, such as in rapidly dividing cancerous cells (Elser et al., 2007b). There can also be a special association between a macromolecule type and a specific cellular structure, e.g., between chlorophyll and chloroplasts, lipids and membranes, or rRNA and ribosomes, resulting in an association between specific metabolic processes and specific elements (Sterner and Elser, 2002; Allen and Gillooly, 2009). Relatively neglected in stoichiometry are storage organelles and associated storage molecules (Table 1). In many organisms—like in most autotrophs and prokaryotes (Lee, 1996; Raven, 1997), storage is a major process that may disrupt the functional link described above between cell stoichiometry and other organelles (Lukas et al., 2011), as well as play many other unforeseen roles (e.g., the many roles of polyphosphates, in Bjorkman, 2014). The role of other cellular components, such as mitochondria and intracellular membranes has been given very little consideration too. Elements beside C, N and P have seldom been analyzed at the cellular level in mainstream biological stoichiometry, outside the realm of unicellulars (Ho et al., 2003). They are however the major concern of the whole field of ionomics and both approaches certainly benefit from each other (Salt et al., 2008), revealing intriguing and potentially meaningful correlations among various elements (Loladze, 2014; Jeyasingh et al., 2017).
Organism
Knowledge of the C:N:P composition of species has greatly expanded since the first systematic measurements done on a couple of zooplankton taxa (Andersen and Hessen, 1991). The current datasets allow generalities to be drawn, as well as comparisons among various taxa, life forms, trophic levels and habitats to be performed (Sardans et al., 2012). However, besides the C, N, and P triptych, knowledge of other elements is still limited even though it is potentially of great ecological significance. For example, the oxygen content (O) of organisms may be a signature of their physiological conditions and of the type of molecules they store (Fagerbakke et al., 1996; Table 1; see also Han et al., 2011). Biases in the groups of organisms represented in datasets also exist, with unicellular algae, terrestrial vascular plants, crustacean zooplankton—mostly herbivorous—and insects overrepresented (but see Amatangelo and Vitousek, 2008; Martinson et al., 2008; Xia et al., 2014; Danger et al., 2016 for examples of recent attempts at evening the balance).
Population
Intra-specific variability in elemental stoichiometry is less well documented than inter-specific variability. There are different ways to measure variability within a population: according to ontogenetic stages (Main et al., 1997; Meunier et al., 2016), driven by a master trait such as body size or reproductive status (Mendez and Karlsson, 2005), controlled by genotypes, environmentally driven (DeMott et al., 2004), or as stochastic inter-individual variability. Teasing apart all these sources of variation is anything but straightforward and only a few attempts have been made so far, revealing a surprisingly large effect of abiotic conditions on supposedly homeostatic organisms (e.g., El-Sabaawi et al., 2012). Intraspecific variability in autotrophs is notoriously large and determined to a great extent by environmental conditions, with little difference between genotypes (Agren, 2008; Agren and Weih, 2012). Interestingly, ontogenetic stage and body size also strongly affect autotroph stoichiometry, reflecting the fundamental links between growth rate, biomolecules and elements as described in the Growth rate hypothesis (Agren, 2008; Elser et al., 2010). Heterotrophs are classically viewed as maintaining a strict stoichiometric homeostasis, but the paradigm is slowly shifting toward the view that the degree of homeostasis differs from strict to loose in heterotrophic species (Persson et al., 2010; Meunier et al., 2014). It is still unclear what determines the degree of homeostasis (Table 1). Potential candidates are ecological factors such as mortality rates (Wang et al., 2012) and the degree of osmotrophy (bacteria, fungi, and flagellates are often highly non-homeostatic, see Godwin and Cotner, 2015; Golz et al., 2015; Danger et al., 2016). Under natural conditions, non-homeostatic species, or conformers—to borrow the concept from physiological studies of homeostasis, are likely to show more intraspecific variability than homeostatic species, or regulators (Meunier et al., 2014). But the latter may still see substantial stoichiometric variability between different life stages or genotypes. It will be important to study the extent of these two sources of intraspecific variability and their effects on populations and nutrient fluxes in ecosystems, likely by integrating elements from the field of developmental biology.
Community
There are numerous studies that examine how elemental composition varies within local communities (Sterner and George, 2000; He et al., 2006; Hattenschwiler et al., 2008). Technical developments in electron microscopy (Gundersen et al., 2002) and microspectroscopy (Hall et al., 2011) allows for such studies in unicellular organisms too. Despite increasing data availability, comparatively few generalities on stoichiometric variability have been drawn (but see Sardans et al., 2012). One reason might be that such studies have not separated scales clearly, mixing data taken from one locality with other data from other localities (Table 1). For example, McGroddy et al. (2004) show that variability in N:P ratios is lower within than between biomes. Phylogeny may contribute to this pattern as was found in plants (Broadley et al., 2004) and insects (Woods et al., 2004). The resource-ratio theory may provide some theoretical underpinning as to why local communities feeding on shared resources might converge toward the same stoichiometry, the one ensuring co-limitation by the multiple shared resources (Cherif and Loreau, 2007; Danger et al., 2008). Functional traits may also explain some of the local variation in ratios. For example, N:P ratios are higher in graminoids and stress-tolerant plants than in forbs and ruderals (Gusewell, 2004). Body size is a recurring trait affecting stoichiometry distribution within local communities (Vanni et al., 2002). Other potential factors affecting inter-specific differences in stoichiometry within and between communities are reviewed in Carnicer et al. (2015). Alternatively, neutral processes might control stoichiometric patterns in communities as they may do for abundance distributions (Chave, 2004). Combination of multiple community ecology theories would certainly improve our understanding of community stoichiometry, since competition theory alone does not explain the high biodiversity found in, for example, phytoplankton communities (Passarge et al., 2006).
Species diversity is a factor that has received special interest recently, both as a factor affecting (Striebel et al., 2009; Abbas et al., 2013) and being affected by stoichiometry (Evans-White et al., 2009; Lewandowska et al., 2016). The study by Guiz et al. (2016) is unique in its attempts at causally explaining both the mean and variation in stoichiometry within local communities and suggests an intriguing convergence in the mean and variance of C:N ratios between plant communities established on different soil fertilities.
Vertical diversity, the number of trophic levels, is another dimension of biodiversity that has garnered some attention from stoichiometry. Causal interactions between food chain length and variation in stoichiometry across trophic levels have been touched upon lately, but without any general conclusions drawn yet (Doi, 2012; Peace, 2015). Few generalities have been found regarding systematic stoichiometric differences across trophic levels, beyond the known fact that herbivores are generally poorer in C than their food, but show similar N:P ratios among habitats (Elser et al., 2000a; Table 1). For secondary consumers and higher trophic levels, studies have found increases, decreases or no change in N and P when climbing the food chain (Lemoine et al., 2014). Missing are more studies measuring the stoichiometric imbalances between predators and their prey in realized interactions (Malzahn et al., 2010; Lemoine et al., 2014), as well as measuring efficiencies and other important metabolic parameters if one wants to infer consequences on nutrient cycling (Doi et al., 2010).
Ecosystem
Generic knowledge of the balance of N and P in major ecosystems has been available for a long time. In terrestrial systems, chronosequence analyses suggest that terrestrial vegetation shifts from N limitation to P limitation as soils age, N2 fixation increases N stocks, and erosion depletes soil P (Vitousek and Farrington, 1997). Hence, it is assumed that P is limiting in the tropics and on old bedrocks, while N is limiting in soils recently sedimented or newly exposed by deglaciation. In oceans, the classical work by Redfield has ingrained the view that total C:N:P ratio in pelagic waters is constant around a mean value of 105:15:1 (Redfield, 1958), with competition between N-fixer and non-fixer phytoplankton as the main regulating process (Lenton and Klausmeier, 2007). In freshwater systems, lakes are assumed to be P limited in temperate areas, because C and N can be incorporated into lakes from their vast atmospheric reservoirs (Schindler, 1977), while N limitation was noted for some large lakes in the tropics (Hecky and Kilham, 1988) and some humic lakes in Scandinavia (Jansson et al., 2001). But with biological stoichiometry, there was a renewed interest in measuring the balance of elements in ecosystems, and the classical paradigms depicted above has seen many challenges (Sterner, 2008). The current image emerging is that of ecosystems that are co-limited, or close to co-limitation, unless high imbalances or perturbations in the relative inputs of elements in the ecosystem occur (Elser et al., 2007a). Recent advances even advocate for light limitation in boreal lakes which are rich in colored dissolved organic matter (Karlsson et al., 2009). Anthropogenic alterations of the biogeochemical cycles may also push ecosystems toward limitation by new elements such as K or Mo (van Groenigen et al., 2006). Hence, the general rules about the balance of elements in ecosystems require constant revision as new studies at the local scale emerge (Table 1). Important lessons from the analysis of an extensive regional lake database (Hessen et al., 2009) are the significance of catchment properties (type and density of terrestrial vegetation), and of the regional context (N or S deposition intensity) in determining lake stoichiometry. The importance of the landscape context should prompt further distantiation from the “lake as a microcosm” paradigm (Jenkins, 2014).
Landscape
The stoichiometric focus in lake studies greatly highlighted the role of the watershed in determining the nutrient status of freshwater bodies (Frost et al., 2009; Hessen et al., 2009). But it also describes a decreasing influence of landscape as the sizes of water bodies increase, with an increasing convergence toward Redfield ratios from lakes to coastal areas to oceanic water bodies (Sterner et al., 2008). Depth is an equally important spatial dimension in aquatic ecosystems. Vertical profiles of nutrients along the water column, and the role of salinity, temperature and turbulence in their generation are classical material of hydrodynamics and aquatic ecology textbooks (Barnes et al.,, 1991). Additions taken from stoichiometry to these classical approaches look very promising already, indicating important interactions between water mixing and stoichiometric properties of organisms (Diehl et al., 2005; Frassl et al., 2014; Table 1).
Terrestrial ecology lags behind in terms of characterizing stoichiometry at the landscape level. Studies searching for differences in elemental availabilities between forests and grasslands for example yielded unresolved contrasting results (Cleveland and Liptzin, 2007; Bond, 2010). There are obvious factors that may affect soil nutrient availabilities between adjacent areas, such as age, vegetation type, or local climate. But there is a lack of knowledge about stoichiometric variation along connected terrestrial ecosystems, for example along a shared hill slope (Porder et al., 2005; Table 1). Stoichiometric approaches proved more beneficial when it comes to vertical profiles in soils, demonstrating a great effect of the biota on the relative distribution of elements along soil depth (Jobbagy and Jackson, 2001). Despite recent efforts, understanding landscape variation in ecosystem stoichiometry remains a challenge (Chadwick and Asner, 2016) and considering supplies from the biosphere is often necessary to balance large-scale elemental budgets (Chadwick et al., 1999).
Biosphere
Recent intensive sampling campaigns, global survey tools and data sharing allow for a better mapping of stoichiometry over continents and oceans (Cleveland and Liptzin, 2007; Elser et al., 2010; Weber and Deutsch, 2010), although P may require more data (Wang et al., 2010). Even less information is available on other elements (Table 1). The emerging picture so far is that local heterogeneity marks elemental distributions on emerged lands, and to a lesser extent in oceans (Key et al., 2004). Atmospheric circulation mixes gases very efficiently in the atmosphere despite persistent spatial and temporal variations (Wunch et al., 2011). But for other elements, like P and sulfur, homogenization is not fast enough compared to their turnover in the atmosphere, and so, deposition is localized around their sources of supply (Garland, 1978; Mahowald et al., 2008). Their radius of impact can still be surprisingly large as, for example, P from Saharan dust is known to fertilize American ecosystems (Okin et al., 2004).
Processes
Processes are generally harder to measure than standing stocks of elements. They often require specific methods and equipment, as well as manipulative experiments, which may be different among elements that must be measured simultaneously in stoichiometric studies. Despite these complications, there are a large number of studies reporting on the various processes affecting the stoichiometry of all biological levels. The picture emerging from these studies, its beacons and shadows, are briefly discussed in this section.
Genetic Expression
Various genetic, biochemical and physiological methods have already been used in order to characterize genetic responses to stoichiometric imbalances (reviewed in Wagner et al., 2013). Studies of the genetic regulation of nutrient limitation has a strong tradition outside of the biological stoichiometry umbrella, mostly from the study of classical model organisms such as the bacterium Escherichia coli, the plant Arabidopsis thaliana and the yeast Saccharomyces cerevisiae (see, e.g., KEGG PATHWAY Database1; Broadley and White, 2009; Broach, 2012). The field seems now ready to move to the next step, from a reductionist to systemic approach, based on approaches such as systems biology and nutrigenomics (Muller and Kersten, 2003; Ruffel et al., 2010; Table 2). In parallel, the stoichiometric approach has provided its own novel perspective, by demonstrating, e.g., a fundamental causal link between the number of rDNA copies in a genome, the length and composition of the associated regulatory IGS regions, the cell ribosomal content, its total P content and somatic growth (Elser et al., 2000c; Weider et al., 2005). Stoichiometry sheds a unique light on the link between the economy of elements in organisms and genome structure by showing that the bulk elemental composition of the genetic material itself matters. Genes answering to a given elemental limitation tend to contain less of the limiting element and to code for proteins that do similarly (Gilbert et al., 2013). The two approaches of nutritional genetics and stoichiometry increasingly exchange methodologies (Wagner et al., 2013), but results and conclusions rarely flow in between. For example, a highly cited, comprehensive investigation of the interactive effects of C and N availability on gene expression regulation (Gutierrez et al., 2007) has gone un-cited in the stoichiometry literature so far.
Ecophysiological Rates
Rates of resource acquisition by organisms as modulated by elemental availability in their environment is an old topic in biology at least since the 1940s (Monod, 1947). The general rule is that of a negative feedback resulting from the availability of a given resource: the lower the availability, the higher the investment of the consumer to acquire it. Stoichiometry has made a substantial contribution to the subject by adding concerns about the potential role of other essential elements. On optimisation grounds, it is expected that organisms should use the excess availability of non-limiting elements to increase their acquisition of the limiting elements until co-limitation by all essential elements is reached (Darchambeau et al., 2003; Klausmeier et al., 2004a; Cherif and Loreau, 2007). However, a number of mechanisms may prevent co-limitation establishing in practice, like limits to physiological adaptation (Klausmeier et al., 2004b). Organism needs are complex, and resource acquisition strategies may have ultimate goals beyond just insuring immediate maximum growth for an organism (Flynn, 2009; Table 2). Besides, variations in the availabilities of various resources tend to be coupled under natural circumstances (Lemoine et al., 2014), sometimes in conjunction with under-appreciated limiting factors, such as water availability (Sardans et al., 2008). Hence multifactorial analyses beyond the usual N and P (sometimes also C) limitation experiments should be extended to include simultaneous variations in combined resources. Besides, the molecules that contain the essential resources may have peripheral chemical properties that otherwise affect growth, e.g., they may be toxic or change the pH of cells (McGrath and Quinn, 2000). They may also play other roles in the metabolism, for example as ions maintaining constant osmolality (e.g., Na, K, and Ca) or as electron donors/acceptors (e.g., O, N, and S). Such side effects have been considered in classical nutritional biology, but mostly ignored in stoichiometry. It is only recently that consideration of factors such as salinity and anoxia in stoichiometry emerged (Marino et al., 2006; Helton et al., 2015; Tadonleke et al., 2016). Even fewer studies have attempted to integrate the role of elements as biomass components with their other chemical functions (but see Payn et al., 2014). Environmental chemistry should thus be better integrated into the stoichiometric analyses of ecophysiological rates (Table 2).
Physiological Rates
Organisms have to match elemental demands set by genetic expression with the ecophysiological rates of resource uptake. They do this through various physiological processes that redistribute elements within the organism or between the organism and its environment, as well as through feedbacks that regulate the genetic expression. Elemental metabolism in primary producers and in some model microorganisms like E. coli and S. cerevisiae is an established part of research. So-called genome-scale metabolic models now offer the possibility to quantitatively predict the effects of changes in resource availabilities or of mutations in genes (e.g., Liu et al., 2010). On the other hand, stoichiometric studies generally concentrate on designing simple models describing the link between elements and growth in autotrophs and osmotrophs (Cherif, 2016). These models are mostly empirically-based and to a great extent divorced from the potential molecular and cellular underpinning mechanisms, which is both a strength and weakness for such models (Flynn, 2008). When it comes to consumers, classical stoichiometric theories assume strict homeostasis of elemental composition, resulting in strong constraints on the physiological fluxes of elements, particularly excretion (Sterner, 1990). This vision of homeostasis in heterotrophs is increasingly challenged (Persson et al., 2010). The quest is now for a mechanistic understanding of the determinants of the degree of homeostasis in organisms (Meunier et al., 2014; Table 2). The main physiological consequence of strict homeostasis is a decrease in growth rate because one element becomes limiting (Sterner and Hessen, 1994). Another consequence is an increase in the costs related to the regulation of the elements in excess, which may also lead to a decrease in growth (Boersma and Elser, 2006). Tolerating changes in stoichiometry by lowering the content of the limiting element, or storing the elements in excess, are strategies that dampen the sensitivity of a species to elemental deficiencies (Mulder and Bowden, 2007; Seidendorf et al., 2010) and may even prove competitively advantageous under both constant and variable environments (Grover, 1991; Grover and Chrzanowski, 2006). The stoichiometry theory has specifically emphasized two ecologically relevant stoichiometric rearrangements: the decrease in P content under P limitation, through a decrease in ribosome numbers (the “Growth rate hypothesis,” see Elser et al., 1996) and the storage of excess C by plants when light and CO2 are non-limiting (the “Light:nutrient hypothesis,” see Sterner et al., 1997). Maintaining health and immunity in the face of a varying environment is a fundamental homeostatic feature, a fact acknowledged by nutrigenomics (Afman and Muller, 2006) but overlooked by stoichiometry (Table 2). If stoichiometric imbalances trigger diseases, then consequences for the organisms may go beyond a decrease in growth rate or reproductive output. More generally, stoichiometric adjustments that affect the life history traits of organisms, should display a variety of demographic and ecological consequences.
Demographic Processes
Classical population biology teaches us that life-history traits determine the demographic processes that set the structure and dynamics of populations (Tuljapurkar and Caswell, 1997). However, classical stoichiometric theories mainly considered the effects of elemental limitation on somatic growth, and more rarely its effects on life history traits (reviewed in Moe et al., 2005). Fecundity was generally found to be affected by poor diet quality (Sterner, 1998; Urabe and Sterner, 2001; Zandona et al., 2011). Few studies focussed on stoichiometry-related mortality (Sterner et al., 1993; Nelson et al., 2001; Declerck et al., 2015; Table 2). Only a couple of studies look into links between stoichiometry and dispersal or migration (Sterner and Schwalbach, 2001; Huberty and Denno, 2006). Empirically, much of this work concentrated on Daphnia and copepods at the expense of other taxa (Sterner and Schulz, 1998; Urabe and Sterner, 2001; Jeyasingh and Weider, 2005; but see Bertram et al., 2006). Hence, the effects of nutrient limitation, more specifically P limitation, on life-history traits in those organisms are reasonably well understood. But we know only of one study that put this information to use in a P-limited population model by Nakazawa (2011). One intriguing result from this study, but in line with most recent population ecology theory (Roos and Persson, 2013), is that, assuming that P limitation affects mainly somatic growth, more P-limited populations should be dominated by P-rich juveniles, and hence be more P-rich (Nakazawa, 2011). More models releasing simplifying assumptions are needed, as well as experimental data outside of the crustacean realm. Other fundamental concepts in population biology, such as demographic stochasticity and density dependence (feedbacks from the population to the organism and cellular levels), are virtually untouched by the stoichiometry theory so far. In short, a stoichiometric theory of demography is sorely lacking (Table 2).
Evolutionary Forces
If sustained over a sufficiently long time, changes in a population structure that are underlain by differences among genomes should result in evolution. Many studies have postulated evolution through adaptation to stoichiometric constraints. Evidence for N limitation shaping the DNA composition of plant genomes, and for P limitation decreasing genome size, P content (Hessen et al., 2010a) and P recycling efficiency (Elser et al., 2000b) has been presented. Evolutionary adaptation requires variability in traits, heritability and correlation to fitness. For a given trait, careful experiments and models examining the mechanisms of natural selection are required. Very few stoichiometric studies committed to examine these mechanisms, either through models (Branco et al., 2010; Yamamichi et al., 2015) or experiments (Declerck et al., 2015; Lind and Jeyasingh, 2015). The absence of mechanistic underpinnings to most evolutionary stoichiometric hypotheses exposes them to criticism (Worman and Kimbrell, 2008; Vieira-Silva et al., 2010; Gunther et al., 2013; Table 2). Moreover, other mechanisms for evolution besides natural selection, such as genetic drift and gene flow, are not yet addressed by any stoichiometric hypotheses (but see Morehouse et al., 2010 for sexual selection).
Ecological Interactions
Ecological interactions are the cornerstones of ecological stoichiometry. The theory established the idea that herbivores are most often limited in their growth by the mineral content of their resources, because most autotrophs are richer in C than their consumers (Elser et al., 2001). Later, mineral limitation was also demonstrated in detritivores (Frost et al., 2002). Subsequently, both deficits and excesses of mineral elements were found to adversely affect the growth rate of consumers (Boersma and Elser, 2006). Secondary consumers were first deemed to be limited by energy, since their prey have similar elemental contents, but given that excess elements can impact growth negatively and that consumer stoichiometry is more variable than previously thought, the jury is still out on whether limitation by resources other than energy is possible (Boersma et al., 2008; Lemoine et al., 2014; Table 2). Besides, imbalance in the stoichiometry of a prey may be associated with other changes in prey quality that may impact predators, an acknowledged but often neglected complication (Mitra and Flynn, 2005). The stoichiometric approach has extended its predictions beyond the immediate effect of stoichiometric imbalances on the growth of the consumer. For example, consumer-driven nutrient recycling predicts that primary consumers should affect the availability of elements at the ecosystem level (Elser and Urabe, 1999) and therefore influence coexistence criteria of primary producers (Grover, 2002); the light:nutrient hypothesis predicts that increased photosynthetic C fixation with increasing light availability should result in widespread mineral limitation throughout the food web (Sterner et al., 1997); a stoichiometric approach to host-pathogen interactions predict that strength of infections should depend on the stoichiometry of the host resources (Smith et al., 2005; Aalto et al., 2015); and stoichiometrically-explicit food webs models suggest food quality-driven changes in trophic cascade strength (Hall et al., 2007). However, those derived predictions often proved difficult to demonstrate unequivocally in theory or experiments (e.g., Daufresne and Loreau, 2001; Sommer et al., 2004; Faithfull et al., 2011; Pulkkinen et al., 2014) probably because the conjectured mechanisms take place at various biological levels and thus need the type of multi-level framework we endeavor to sketch here. Another explanation is that neglecting non-trophic interactions (Kefi et al., 2012) and feedback loops between ecological interactions and physiological rates may have potent stoichiometric effects on higher-level processes (Leroux and Schmitz, 2015).
Recycling Rates
A clear conceptual advance gained from the stoichiometry theory is the connection between stoichiometric consumer-to-resource imbalances and the ratios of elements recycled by consumers (Sterner, 1990). This approach, however, has abstracted release rates as spatially, temporally and chemically uniform. But recycling by consumers is known to be spatially localized (Augustine, 2003), temporally fluctuating (Blackburn et al., 1998) and chemically variable (Anderson et al., 2005). This variability bears important consequences for the overall effects of recycling (Kato et al., 2007; Ramin et al., 2012; Table 2). Recycling rates are predominantly metabolic rates (i.e., excretion and egestion rates). As such, body size and temperature are important rate controllers in concert with stoichiometry (Cross et al., 2015). Recent integration of both stoichiometry and metabolic ecology approaches generally improved rate predictions (Vanni and McIntyre, 2016). The overall chemical conditions, such as anoxia levels, also interact with stoichiometry to determine recycling (Steenbergh et al., 2013). Oxygen minima zones are thus likely to affect and be affected by the recycling rates of essential elements (Stramma et al., 2010; Penn et al., 2016). This example points to the importance of considering the potential feedbacks between the chemical environment and recycling (Table 2). Research in biodiversity-ecosystem functioning relationships has otherwise shown that species diversity affects elemental rates within ecosystems (Hooper et al., 2005), as well as biomass production and stoichiometry of primary producers (Striebel et al., 2009). However, interactive effects of stoichiometry and biodiversity on recycling rates are still poorly understood and in need of further development (Hillebrand et al., 2014). In the long term, it has been shown that it is recycling of the forms that are not available to other organisms that determine the impact of a consumer on nutrient availability in its ecosystem (Paterson et al., 2002; Vanni et al., 2013). This impact depends on the quantity and quality of matter that it subtracts from its environment through, e.g., sedimented feces, recalcitrant carcasses or emigration. Potential control of the consumer on the inputs of elements in the ecosystem needs to be considered similarly (e.g., through N fixation, immigration). Hence, the effects of recycling are intimately related to transfer rates among ecosystems, because elements that are removed from an ecosystem are obligatorily added to another ecosystem thus affecting both ecosystems.
Transfer Rates
Translocation of elements among ecosystems has been an early scientific concern, defining the field of biogeochemistry (Gorham, 1991). However, the role of living organisms in the coupling of the fluxes of multiple elements has only been conceptualized and generalized to all ecosystems with the birth of the stoichiometric approach (Sterner and Elser, 2002). On the theoretical level, Schade et al. (2005) attempted to model the interaction of ecosystems through transfers of elements among them with an extension of the classical resource-ratio theory (Tilman, 1982), insisting on the importance of dispersing organisms and of boundaries in altering the stoichiometry of the transferred material and of the recipient ecosystem. Usage of this framework remains limited however, probably because of a relative paucity in data reporting the transfer rates of multiple elements simultaneously (reviewed in Sitters et al., 2015; Table 2). Other unifying frameworks, such as meta-ecosystems, are worth exploring as well (Loreau et al., 2003; Manzoni and Porporato, 2011; Marleau et al., 2015).
General Circulation
Shortcuts to localized transfers between adjacent ecosystems take place when elements are redistributed on larger scales by the atmospheric and oceanic circulations. Multiple processes put elements in general circulation: erosion, evapotranspiration, respiration, and most important, human-mediated processes such as fossil fuel burning. Other processes ensure that those elements fall back on local ecosystems. Some are physical (atmospheric deposition, precipitation, upwelling), others are mediated by living organisms (photosynthesis, N-fixation, fertilizer production by humans). Those processes are rarely studied together, or for multiple elements simultaneously (Table 2). Because of their sheer biomass and level of activity, plants (Wassen et al., 2013) and humans (Penuelas et al., 2013) are the biotic components that most affect the general circulation of elements. Explicit stoichiometric considerations increased the predictability of general circulation models that include the potential effects of these biotic drivers on the dynamics of the biosphere (Thornton et al., 2009; Penuelas et al., 2013).
Conclusion: A Roadmap for a Multilevel, Comprehensive Theory of Stoichiometry
Our framework provides a roadmap of the way a given stoichiometric imbalance at a given biological level, or alteration in a given stoichiometric process can cascade to other biological levels by affecting higher- or lower-level processes. The survey of the literature structured by our framework, although non-exhaustive, shows that the theory has proved astonishingly vigorous despite its relatively young age, as judged by its degree of completeness and development. But we also identified, for each level and each process of the framework, areas in need of completion, development, or articulation with other relevant non-stoichiometric theories (see Tables 1, 2 for a summary). Stoichiometric theories for population dynamics and community composition appear to us as most urgently needed. Populations and communities obey constraints that are beyond physiology. These two biological levels may thus result in departures from the physiological rules that are used by stoichiometry theories to predict the flows of multiple elements. Various disciplines have already developed reliable theories, concepts and methodologies related to these areas in need of development, which are highly relevant to the stoichiometric approach. But lack of familiarity with other fields from the stoichiometry side, and lack of a comprehensive view on the stoichiometric approach on the other side, is likely to hinder integration, unless the links between fields are explicitly laid out, like in our framework.
By highlighting many areas in need of improvement, our framework indicates that the stoichiometry approach has not yet delivered a predictive theory of elemental transfers within the biosphere. But, we have every hope that our framework will help the theory reach a level of maturity such that it will be able to consistently relate local, microscopic processes to global, macroscopic processes. Current general circulation models often flagrantly ignore lower-scale biological processes for lack of a proper methodology, potentially restraining our capacity to accurately predict future global change. Our framework is one step in this direction.
Author Contributions
All authors contributed to the conception and design of the study and to the selection, analysis and interpretation of the relevant literature. MC coordinated the writing of the manuscript. CF, CM, JS, WU, and FR revised the manuscript critically. All authors approve the manuscript in its current form and agree to be accountable for all aspects of the work.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
MC was supported by the strong research environment “Ecosystem Change” of Umeå University. JS was financially supported by a grant from the Research Foundation Flanders (FWO). CF was financially supported by a postdoctoral grant from Vetenskapsrådet (637-2013-7449). We thank Michel Loreau, Claire de Mazancourt, and Göran Englund for valuable comments on early versions of the manuscript.
Footnotes
1. ^KEGG PATHWAY Database. Available online at: http://www.genome.jp/kegg/pathway.html (Online).
References
Aalto, S. L., Decaestecker, E., and Pulkkinen, K. (2015). A three-way perspective of stoichiometric changes on host-parasite interactions. Trends Parasitol. 31, 333–340. doi: 10.1016/j.pt.2015.04.005
Abbas, M., Ebeling, A., Oelmann, Y., Ptacnik, R., Roscher, C., Weigelt, A., et al. (2013). Biodiversity effects on plant stoichiometry. PLoS ONE 8:e58179. doi: 10.1371/journal.pone.0058179
Acquisti, C., Elser, J. J., and Kumar, S. (2009). Ecological nitrogen limitation shapes the DNA composition of plant genomes. Mol. Biol. Evol. 26, 953–956. doi: 10.1093/molbev/msp038
Afman, L., and Muller, M. (2006). Nutrigenomics: from molecular nutrition to prevention of disease. J. Am. Diet. Assoc. 106, 569–576. doi: 10.1016/j.jada.2006.01.001
Agren, G. I. (2008). Stoichiometry and nutrition of plant growth in natural communities. Annu. Rev. Ecol. Evol. S. 39, 153–170. doi: 10.1146/annurev.ecolsys.39.110707.173515
Agren, G. I., and Weih, M. (2012). Plant stoichiometry at different scales: element concentration patterns reflect environment more than genotype. New Phytol. 194, 944–952. doi: 10.1111/j.1469-8137.2012.04114.x
Allen, A. P., and Gillooly, J. F. (2009). Towards an integration of ecological stoichiometry and the metabolic theory of ecology to better understand nutrient cycling. Ecol. Lett. 12, 369–384. doi: 10.1111/j.1461-0248.2009.01302.x
Allen, T. F. H., and Hoekstra, T. W. (1990). The Confusion between scale-defined levels and conventional levels of organization in ecology. J. Vegetation Sci. 1, 5–12. doi: 10.2307/3236048
Allen, T. F. H., and Hoekstra, T. W. (1993). Toward a Unified Ecology. New York, NY: Columbia University Press.
Amatangelo, K. L., and Vitousek, P. M. (2008). Stoichiometry of ferns in Hawaii: implications for nutrient cycling. Oecologia 157, 619–627. doi: 10.1007/s00442-008-1108-9
Andersen, T., and Hessen, D. O. (1991). Carbon, nitrogen, and phosphorus-content of fresh-water zooplankton. Limnol. Oceanogr. 36, 807–814. doi: 10.4319/lo.1991.36.4.0807
Anderson, T. R., Hessen, D. O., Elser, J. J., and Urabe, J. (2005). Metabolic stoichiometry and the fate of excess carbon and nutrients in consumers. Am. Nat. 165, 1–15. doi: 10.1086/426598
Augustine, D. J. (2003). Long-term, livestock-mediated redistribution of nitrogen and phosphorus in an East African savanna. J. Appl. Ecol. 40, 137–149. doi: 10.1046/j.1365-2664.2003.00778.x
Barnes, R. S. K., and Mann, K. H., and Wiley InterScience (Online service) (1991). Fundamentals of Aquatic Ecology. Oxford; Boston, MA: Blackwell.
Bertram, S. M., Schade, J. D., and Elser, J. J. (2006). Signalling and phosphorus: correlations between mate signalling effort and body elemental composition in crickets. Anim. Behav. 72, 899–907. doi: 10.1016/j.anbehav.2006.02.012
Bjorkman, K. M. (2014). Polyphosphate goes from pedestrian to prominent in the marine P-cycle. Proc. Natl. Acad. Sci. U.S.A. 111, 7890–7891. doi: 10.1073/pnas.1407195111
Blackburn, N., Fenchel, T., and Mitchell, J. (1998). Microscale nutrient patches in planktonic habitats shown by chemotactic bacteria. Science 282, 2254–2256. doi: 10.1126/science.282.5397.2254
Boersma, M., Aberle, N., Hantzsche, F. M., Schoo, K. L., Wiltshire, K. H., and Malzahn, A. M. (2008). Nutritional limitation travels up the food chain. Int. Rev. Hydrobiol. 93, 479–488. doi: 10.1002/iroh.200811066
Boersma, M., and Elser, J. J. (2006). Too much of a good thing: on stoichiometrically balanced diets and maximal growth. Ecology 87, 1325–1330. doi: 10.1890/0012-9658(2006)87[1325:TMOAGT]2.0.CO;2
Bond, W. J. (2010). Do nutrient-poor soils inhibit development of forests? A nutrient stock analysis. Plant Soil 334, 47–60. doi: 10.1007/s11104-010-0440-0
Bragg, J. G., and Hyder, C. L. (2004). Nitrogen versus carbon use in prokaryotic genomes and proteomes. Proc. R. Soc. B Biol. Sci. 271, S374–S377. doi: 10.1098/rsbl.2004.0193
Branco, P., Stomp, M., Egas, M., and Huisman, J. (2010). Evolution of nutrient uptake reveals a trade-off in the ecological stoichiometry of plant-herbivore interactions. Am. Nat. 176, E162–E176. doi: 10.1086/657036
Broach, J. R. (2012). Nutritional control of growth and development in yeast. Genetics 192:73. doi: 10.1534/genetics.111.135731
Broadley, M. R., Bowen, H. C., Cotterill, H. L., Hammond, J. P., Meacham, M. C., Mead, A., et al. (2004). Phylogenetic variation in the shoot mineral concentration of angiosperms. J. Exp. Bot. 55, 321–336. doi: 10.1093/jxb/erh002
Carnicer, J., Sardans, J., Stefanescu, C., Ubach, A., Bartrons, M., Asensio, D., et al. (2015). Global biodiversity, stoichiometry and ecosystem function responses to human-induced C-N-P imbalances. J. Plant Physiol. 172, 82–91. doi: 10.1016/j.jplph.2014.07.022
Chadwick, K. D., and Asner, G. P. (2016). Tropical soil nutrient distributions determined by biotic and hillslope processes. Biogeochemistry 127, 273–289. doi: 10.1007/s10533-015-0179-z
Chadwick, O. A., Derry, L. A., Vitousek, P. M., Huebert, B. J., and Hedin, L. O. (1999). Changing sources of nutrients during four million years of ecosystem development. Nature 397, 491–497. doi: 10.1038/17276
Chave, J. (2004). Neutral theory and community ecology. Ecol. Lett. 7, 241–253. doi: 10.1111/j.1461-0248.2003.00566.x
Cherif, M. (2016). Stoichiometry and population growth in osmotrophs and non-osmotrophs. eLS 1–6. doi: 10.1002/9780470015902.a0026353
Cherif, M., and Loreau, M. (2007). Stoichiometric constraints on resource use, competitive interactions, and elemental cycling in microbial decomposers. Am. Nat. 169, 709–724. doi: 10.1086/516844
Cleveland, C. C., and Liptzin, D. (2007). C: N: P stoichiometry in soil: is there a “Redfield ratio” for the microbial biomass? Biogeochemistry 85, 235–252. doi: 10.1007/s10533-007-9132-0
Cross, W. F., Hood, J. M., Benstead, J. P., Huryn, A. D., and Nelson, D. (2015). Interactions between temperature and nutrients across levels of ecological organization. Glob. Chang. Biol. 21, 1025–1040. doi: 10.1111/gcb.12809
Danger, M., Daufresne, T., Lucas, F., Pissard, S., and Lacroix, G. (2008). Does Liebig's law of the minimum scale up from species to communities? Oikos 117, 1741–1751. doi: 10.1111/j.1600-0706.2008.16793.x
Danger, M., Gessner, M. O., and Barlocher, F. (2016). Ecological stoichiometry of aquatic fungi: current knowledge and perspectives. Fungal Ecol. 19, 100–111. doi: 10.1016/j.funeco.2015.09.004
Darchambeau, F., Faerovig, P. J., and Hessen, D. O. (2003). How Daphnia copes with excess carbon in its food. Oecologia 136, 336–346. doi: 10.1007/s00442-003-1283-7
Daufresne, T., and Loreau, M. (2001). Plant-herbivore interactions and ecological stoichiometry: when do herbivores determine plant nutrient limitation? Ecol. Lett. 4, 196–206. doi: 10.1046/j.1461-0248.2001.00210.x
Declerck, S. A. J., Malo, A. R., Diehl, S., Waasdorp, D., Lemmen, K. D., Proios, K., et al. (2015). Rapid adaptation of herbivore consumers to nutrient limitation: eco-evolutionary feedbacks to population demography and resource control. Ecol. Lett. 18, 553–562. doi: 10.1111/ele.12436
DeMott, W. R., Pape, B. J., and Tessier, A. J. (2004). Patterns and sources of variation in Daphnia phosphorus content in nature. Aquatic Ecol. 38, 433–440. doi: 10.1023/B:AECO.0000035183.53389.66
Diehl, S., Berger, S., and Wohrl, R. (2005). Flexible nutrient stoichiometry mediates environmental influences, on phytoplankton and its resources. Ecology 86, 2931–2945. doi: 10.1890/04-1512
Doi, H. (2012). Resource productivity and availability impacts for food-chain length. Ecol. Res. 27, 521–527. doi: 10.1007/s11284-012-0941-9
Doi, H., Cherif, M., Iwabuchi, T., Katano, I., Stegen, J. C., and Striebel, M. (2010). Integrating elements and energy through the metabolic dependencies of gross growth efficiency and the threshold elemental ratio. Oikos 119, 752–765. doi: 10.1111/j.1600-0706.2009.18540.x
El-Sabaawi, R. W., Kohler, T. J., Zandona, E., Travis, J., Marshall, M. C., Thomas, S. A., et al. (2012). Environmental and organismal predictors of intraspecific variation in the stoichiometry of a neotropical freshwater fish. PLoS ONE 7:e32713. doi: 10.1371/journal.pone.0032713
Elser, J. (2006). Biological stoichiometry: a chemical bridge between ecosystem ecology and evolutionary biology. Am. Nat. 168, S25–S35. doi: 10.1086/509048
Elser, J. J., Acquisti, C., and Kumar, S. (2011). Stoichiogenomics: the evolutionary ecology of macromolecular elemental composition. Trends Ecol. Evol. 26, 38–44. doi: 10.1016/j.tree.2010.10.006
Elser, J. J., Bracken, M. E. S., Cleland, E. E., Gruner, D. S., Harpole, W. S., Hillebrand, H., et al. (2007a). Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol. Lett. 10, 1135–1142. doi: 10.1111/j.1461-0248.2007.01113.x
Elser, J. J., Dobberfuhl, D. R., MacKay, N. A., and Schampel, J. H. (1996). Organism size, life history, and N:P stoichiometry. Bioscience 46, 674–684. doi: 10.2307/1312897
Elser, J. J., Elser, M. M., Mackay, N. A., and Carpenter, S. R. (1988). Zooplankton-mediated transitions between N-limited and P-limited algal growth. Limnol. Oceanogr. 33, 1–14. doi: 10.4319/lo.1988.33.1.0001
Elser, J. J., Fagan, W. F., Denno, R. F., Dobberfuhl, D. R., Folarin, A., Huberty, A., et al. (2000a). Nutritional constraints in terrestrial and freshwater food webs. Nature 408, 578–580. doi: 10.1038/35046058
Elser, J. J., Fagan, W. F., Kerkhoff, A. J., Swenson, N. G., and Enquist, B. J. (2010). Biological stoichiometry of plant production: metabolism, scaling and ecological response to global change. New Phytol. 186, 593–608. doi: 10.1111/j.1469-8137.2010.03214.x
Elser, J. J., Hayakawa, K., and Urabe, J. (2001). Nutrient limitation reduces food quality for zooplankton: Daphnia response to seston phosphorus enrichment. Ecology 82, 898–903. doi: 10.1890/0012-9658(2001)082[0898:NLRFQF]2.0.CO;2
Elser, J. J., Kyle, M. M., Smith, M. S., and Nagy, J. D. (2007b). Biological stoichiometry in human cancer. PLoS ONE 2:e1028. doi: 10.1371/journal.pone.0001028
Elser, J. J., Nagy, J. D., and Kuang, Y. (2003). Biological stoichiometry: an ecological perspective on tumor dynamics. Bioscience 53, 1112–1120. doi: 10.1641/0006-3568(2003)053[1112:BSAEPO]2.0.CO;2
Elser, J. J., O'Brien, W. J., Dobberfuhl, D. R., and Dowling, T. E. (2000b). The evolution of ecosystem processes: growth rate and elemental stoichiometry of a key herbivore in temperate and arctic habitats. J. Evol. Biol. 13, 845–853. doi: 10.1046/j.1420-9101.2000.00215.x
Elser, J. J., Sterner, R. W., Gorokhova, E., Fagan, W. F., Markow, T. A., Cotner, J. B., et al. (2000c). Biological stoichiometry from genes to ecosystems. Ecol. Lett. 3, 540–550. doi: 10.1046/j.1461-0248.2000.00185.x
Elser, J. J., and Urabe, J. (1999). The stoichiometry of consumer-driven nutrient recycling: theory, observations, and consequences. Ecology 80, 735–751. doi: 10.1890/0012-9658(1999)080[0735:TSOCDN]2.0.CO;2
Elser, J. J., Watts, J., Schampel, J. H., and Farmer, J. (2006). Early Cambrian food webs on a trophic knife-edge? A hypothesis and preliminary data from a modern stromatolite-based ecosystem. Ecol. Lett. 9, 292–300. doi: 10.1111/j.1461-0248.2005.00873.x
Evans, J. R. (1989). Photosynthesis and nitrogen relationships in leaves of C-3 plants. Oecologia 78, 9–19. doi: 10.1007/BF00377192
Evans-White, M. A., Dodds, W. K., Huggins, D. G., and Baker, D. S. (2009). Thresholds in macroinvertebrate biodiversity and stoichiometry across water-quality gradients in Central Plains (USA) streams. J. North Am. Benthol. Soc. 28, 855–868. doi: 10.1899/08-113.1
Fagerbakke, K. M., Heldal, M., and Norland, S. (1996). Content of carbon, nitrogen, oxygen, sulfur and phosphorus in native aquatic and cultured bacteria. Aquat. Microb. Ecol. 10, 15–27. doi: 10.3354/ame010015
Faithfull, C. L., Wenzel, A., Vrede, T., and Bergstrom, A. K. (2011). Testing the light: nutrient hypothesis in an oligotrophic boreal lake. Ecosphere 2, 1–15. doi: 10.1890/ES11-00223.1
Field, C. B., and Mooney, H. A. (1986). “The photosynthesis-nitrogen relationship in wild plants,” in On the Economy of Plant Form and Function, ed T. J. Givnish (Cambridge: Cambridge University Press), 25–55.
Flynn, K. J. (2008). The importance of the form of the quota curve and control of non-limiting nutrient transport in phytoplankton models. J. Plankton Res. 30, 423–438. doi: 10.1093/plankt/fbn007
Flynn, K. J. (2009). Going for the slow burn: why should possession of a low maximum growth rate be advantageous for microalgae? Plant Ecol. Divers. 2, 179–189. doi: 10.1080/17550870903207268
Flynn, K. J. (2010). Ecological modelling in a sea of variable stoichiometry: dysfunctionality and the legacy of Redfield and Monod. Prog. Oceanogr. 84, 52–65. doi: 10.1016/j.pocean.2009.09.006
Frassl, M. A., Rothhaupt, K. O., and Rinke, K. (2014). Algal internal nutrient stores feedback on vertical phosphorus distribution in large lakes. J. Great Lakes Res. 40, 162–172. doi: 10.1016/j.jglr.2013.11.001
Frost, P. C., Kinsman, L. E., Johnston, C. A., and Larson, J. H. (2009). Watershed discharge modulates relationships between landscape components and nutrient ratios in stream seston. Ecology 90, 1631–1640. doi: 10.1890/08-1534.1
Frost, P. C., Stelzer, R. S., Lamberti, G. A., and Elser, J. J. (2002). Ecological stoichiometry of trophic interactions in the benthos: understanding the role of C: N: P ratios in lentic and lotic habitats. J. North Am. Benthol. Soc. 21, 515–528. doi: 10.2307/1468427
Garland, J. A. (1978). “Dry and wet removal of sulphur from the atmosphere,” in Sulfur in the Atmosphere (Pergamon), 349–362.
Gilbert, J. D. J., Acquisti, C., Martinson, H. M., Elser, J. J., Kumar, S., and Fagan, W. F. (2013). GRASP [Genomic Resource Access for Stoichioproteomics]: comparative explorations of the atomic content of 12 Drosophila proteomes. BMC Genomics 14:599. doi: 10.1186/1471-2164-14-599
Godwin, C. M., and Cotner, J. B. (2015). Aquatic heterotrophic bacteria have highly flexible phosphorus content and biomass stoichiometry. Isme J. 9, 2324–2327. doi: 10.1038/ismej.2015.34
Golz, A. L., Burian, A., and Winder, M. (2015). Stoichiometric regulation in micro- and mesozooplankton. J. Plankton Res. 37, 293–305. doi: 10.1093/plankt/fbu109
Gorham, E. (1991). Biogeochemistry—its origins and development. Biogeochemistry 13, 199–239. doi: 10.1007/BF00002942
Grover, J. P. (1991). Resource competition in a variable environment—phytoplankton growing according to the variable-internal-stores model. Am. Nat. 138, 811–835. doi: 10.1086/285254
Grover, J. P. (2002). Stoichiometry, herbivory and competition for nutrients: simple models based on planktonic ecosystems. J. Theor. Biol. 214, 599–618. doi: 10.1006/jtbi.2001.2488
Grover, J. P., and Chrzanowski, T. H. (2006). Stoichiometry and growth kinetics in the “smallest zooplankton”—phagotrophic flagellates. Arch. Hydrobiol. 167, 467–487. doi: 10.1127/0003-9136/2006/0167-0467
Guiz, J., Hillebrand, H., Borer, E. T., Abbas, M., Ebeling, A., Weigelt, A., et al. (2016). Long-term effects of plant diversity and composition on plant stoichiometry. Oikos 125, 613–621. doi: 10.1111/oik.02504
Gundersen, K., Heldal, M., Norland, S., Purdie, D. A., and Knap, A. H. (2002). Elemental C, N, and P cell content of individual bacteria collected at the Bermuda Atlantic Time-Series Study (BATS) site. Limnol. Oceanogr. 47, 1525–1530. doi: 10.4319/lo.2002.47.5.1525
Gunther, T., Lampei, C., and Schmid, K. J. (2013). Mutational bias and gene conversion affect the intraspecific nitrogen stoichiometry of the Arabidopsis thaliana transcriptome. Mol. Biol. Evol. 30, 561–568. doi: 10.1093/molbev/mss249
Gusewell, S. (2004). N: P ratios in terrestrial plants: variation and functional significance. New Phytol. 164, 243–266. doi: 10.1111/j.1469-8137.2004.01192.x
Gutierrez, R. A., Lejay, L. V., Dean, A., Chiaromonte, F., Shasha, D. E., and Coruzzi, G. M. (2007). Qualitative network models and genome-wide expression data define carbon/nitrogen-responsive molecular machines in Arabidopsis. Genome Biol. 8:R7. doi: 10.1186/gb-2007-8-1-r7
Hall, E. K., Singer, G. A., Polzl, M., Hammerle, I., Schwarz, C., Daims, H., et al. (2011). Looking inside the box: using Raman microspectroscopy to deconstruct microbial biomass stoichiometry one cell at a time. Isme J. 5, 196–208. doi: 10.1038/ismej.2010.115
Hall, S. R. (2009). Stoichiometrically Explicit Food Webs: feedbacks between resource supply, elemental constraints, and species diversity. Ann. Rev. Ecol. Evol. Syst. 40, 503–528. doi: 10.1146/annurev.ecolsys.39.110707.173518
Hall, S. R., Shurin, J. B., Diehl, S., and Nisbet, R. M. (2007). Food quality, nutrient limitation of secondary production, and the strength of trophic cascades. Oikos 116, 1128–1143. doi: 10.1111/j.0030-1299.2007.15875.x
Han, W. X., Fang, J. Y., Reich, P. B., Woodward, F. I., and Wang, Z. H. (2011). Biogeography and variability of eleven mineral elements in plant leaves across gradients of climate, soil and plant functional type in China. Ecol. Lett. 14, 788–796. doi: 10.1111/j.1461-0248.2011.01641.x
Hattenschwiler, S., Aeschlimann, B., Couteaux, M. M., Roy, J., and Bonal, D. (2008). High variation in foliage and leaf litter chemistry among 45 tree species of a neotropical rainforest community. New Phytol. 179, 165–175. doi: 10.1111/j.1469-8137.2008.02438.x
He, J. S., Fang, J. Y., Wang, Z. H., Guo, D. L., Flynn, D. F. B., and Geng, Z. (2006). Stoichiometry and large-scale patterns of leaf carbon and nitrogen in the grassland biomes of China. Oecologia 149, 115–122. doi: 10.1007/s00442-006-0425-0
Hecky, R. E., and Kilham, P. (1988). Nutrient Limitation of phytoplankton in fresh-water and marine environments - a review of recent-evidence on the effects of enrichment. Limnol. Oceanogr. 33, 796–822.
Helton, A. M., Ardon, M., and Bernhardt, E. S. (2015). Thermodynamic constraints on the utility of ecological stoichiometry for explaining global biogeochemical patterns. Ecol. Lett. 18, 1049–1056. doi: 10.1111/ele.12487
Hessen, D. O., Andersen, T., Larsen, S., Skjelkvale, B. L., and de Wit, H. A. (2009). Nitrogen deposition, catchment productivity, and climate as determinants of lake stoichiometry. Limnol. Oceanogr. 54, 2520–2528. doi: 10.4319/lo.2009.54.6_part_2.2520
Hessen, D. O., Elser, J. J., Sterner, R. W., and Urabe, J. (2013). Ecological stoichiometry: an elementary approach using basic principles. Limnol. Oceanogr. 58, 2219–2236. doi: 10.4319/lo.2013.58.6.2219
Hessen, D. O., Jeyasingh, P. D., Neiman, M., and Weider, L. J. (2010a). Genome streamlining and the elemental costs of growth. Trends Ecol. Evol. 25, 75–80. doi: 10.1016/j.tree.2009.08.004
Hessen, D. O., Jeyasingh, P. D., Neiman, M., and Weider, L. J. (2010b). Genome streamlining in prokaryotes versus eukaryotes response. Trends Ecol. Evol. 25, 320–321. doi: 10.1016/j.tree.2010.03.003
Hillebrand, H., Cowles, J. M., Lewandowska, A., Van de Waal, D. B., and Plum, C. (2014). Think ratio! A stoichiometric view on biodiversity-ecosystem functioning research. Basic Appl. Ecol. 15, 465–474. doi: 10.1016/j.baae.2014.06.003
Ho, T. Y., Quigg, A., Finkel, Z. V., Milligan, A. J., Wyman, K., Falkowski, P. G., et al. (2003). The elemental composition of some marine phytoplankton. J. Phycol. 39, 1145–1159. doi: 10.1111/j.0022-3646.2003.03-090.x
Hooper, D. U., Chapin, F. S., Ewel, J. J., Hector, A., Inchausti, P., Lavorel, S., et al. (2005). Effects of biodiversity on ecosystem functioning: a consensus of current knowledge. Ecol. Monogr. 75, 3–35. doi: 10.1890/04-0922
Huberty, A. F., and Denno, R. F. (2006). Consequences of nitrogen and phosphorus limitation for the performance of two planthoppers with divergent life-history strategies. Oecologia 149, 444–455. doi: 10.1007/s00442-006-0462-8
Irschick, D. J., Fox, C., Thompson, K., Knapp, A., Baker, L., and Meyer, J. (2013). Functional ecology: integrative research in the modern age of ecology. Funct. Ecol. 27, 1–4. doi: 10.1111/1365-2435.12037
Jansson, M., Bergstrom, A. K., Drakare, S., and Blomqvist, P. (2001). Nutrient limitation of bacterioplankton and phytoplankton in humic lakes in northern Sweden. Freshw. Biol. 46, 653–666. doi: 10.1046/j.1365-2427.2001.00703.x
Jenkins, D. G. (2014). Lakes and rivers as microcosms, version 2.0. J. Limnol. 73, 20–32. doi: 10.4081/jlimnol.2014.797
Jeyasingh, P. D., Goos, J. M., Thompson, S. K., Godwin, C. M., and Cotner, J. B. (2017). Ecological stoichiometry beyond redfield: an ionomic perspective on elemental homeostasis. Front. Microbiol. 8:722. doi: 10.3389/fmicb.2017.00722
Jeyasingh, P. D., and Weider, L. J. (2005). Phosphorus availability mediates plasticity in life-history traits and predator-prey interactions in Daphnia. Ecol. Lett. 8, 1021–1028. doi: 10.1111/j.1461-0248.2005.00803.x
Jobbagy, E. G., and Jackson, R. B. (2001). The distribution of soil nutrients with depth: global patterns and the imprint of plants. Biogeochemistry 53, 51–77. doi: 10.1023/A:1010760720215
Karlsson, J., Bystrom, P., Ask, J., Ask, P., Persson, L., and Jansson, M. (2009). Light limitation of nutrient-poor lake ecosystems. Nature 460, 506–580. doi: 10.1038/nature08179
Kato, S., Urabe, J., and Kawata, M. (2007). Effects of temporal and spatial heterogeneities created by consumer-driven nutrient recycling on algal diversity. J. Theor. Biol. 245, 364–377. doi: 10.1016/j.jtbi.2006.10.012
Kearns, L. P., and Sigee, D. C. (1979). High-levels of transition-metals in dinoflagellate chromosomes. Experientia 35, 1332–1334. doi: 10.1007/BF01963991
Kefi, S., Berlow, E. L., Wieters, E. A., Navarrete, S. A., Petchey, O. L., Wood, S. A., et al. (2012). More than a meal…integrating non-feeding interactions into food webs. Ecol. Lett. 15, 291–300. doi: 10.1111/j.1461-0248.2011.01732.x
Key, R. M., Kozyr, A., Sabine, C. L., Lee, K., Wanninkhof, R., Bullister, J. L., et al. (2004). A global ocean carbon climatology: results from Global Data Analysis Project (GLODAP). Global Biogeochem. Cycles 18, 1–23. doi: 10.1029/2004GB002247
Klausmeier, C. A., Litchman, E., Daufresne, T., and Levin, S. A. (2004a). Optimal nitrogen-to-phosphorus stoichiometry of phytoplankton. Nature 429, 171–174. doi: 10.1038/nature02454
Klausmeier, C. A., Litchman, E., and Levin, S. A. (2004b). Phytoplankton growth and stoichiometry under multiple nutrient limitation. Limnol. Oceanogr. 49, 1463–1470. doi: 10.4319/lo.2004.49.4_part_2.1463
Lee, S. Y. (1996). Bacterial polyhydroxyalkanoates. Biotechnol. Bioeng. 49, 1–14. doi: 10.1002/(SICI)1097-0290(19960105)49:1<1::AID-BIT1>3.0.CO;2-P
Lemoine, N. P., Giery, S. T., and Burkepile, D. E. (2014). Differing nutritional constraints of consumers across ecosystems. Oecologia 174, 1367–1376. doi: 10.1007/s00442-013-2860-z
Lenton, T. M., and Klausmeier, C. A. (2007). Biotic stoichiometric controls on the deep ocean N: P ratio. Biogeosciences 4, 353–367. doi: 10.5194/bg-4-353-2007
Leroux, S. J., and Schmitz, O. J. (2015). Predator-driven elemental cycling: the impact of predation and risk effects on ecosystem stoichiometry. Ecol. Evol. 5, 4976–4988. doi: 10.1002/ece3.1760
Lewandowska, A. M., Biermann, A., Borer, E. T., Cebrian-Piqueras, M. A., Declerck, S. A., De Meester, L., et al. (2016). The influence of balanced and imbalanced resource supply on biodiversity-functioning relationship across ecosystems. Philos. Trans. R. Soc. Lond,. B Biol. Sci. 371:20150283. doi: 10.1098/rstb.2015.0283
Liebig, J., and Playfair, L. P. (1840). Organic Chemistry in its Applications to Agriculture and Physiology. eds J. Liebig (London: Printed for Taylor and Walton).
Lind, P. R., and Jeyasingh, P. D. (2015). Genotypic differences in phosphorus use physiology in producers (Chlamydomonas reinhardtii) and consumers (Daphnia pulex) interact to alter primary and secondary production. Evol. Ecol. 29, 551–563. doi: 10.1007/s10682-015-9760-0
Liu, L. M., Agren, R., Bordel, S., and Nielsen, J. (2010). Use of genome-scale metabolic models for understanding microbial physiology. FEBS Lett. 584, 2556–2564. doi: 10.1016/j.febslet.2010.04.052
Loladze, I. (2014). Hidden shift of the ionome of plants exposed to elevated CO2 depletes minerals at the base of human nutrition. Elife 3:e02245. doi: 10.7554/eLife.02245
Loladze, I., and Elser, J. J. (2011). The origins of the redfield nitrogen-to-phosphorus ratio are in a homoeostatic protein-to-rRNA ratio. Ecol. Lett. 14, 244–250. doi: 10.1111/j.1461-0248.2010.01577.x
Loreau, M., Mouquet, N., and Holt, R. D. (2003). Meta-ecosystems: a theoretical framework for a spatial ecosystem ecology. Ecol. Lett. 6, 673–679. doi: 10.1046/j.1461-0248.2003.00483.x
Lukas, M., Sperfeld, E., and Wacker, A. (2011). Growth rate hypothesis does not apply across colimiting conditions: cholesterol limitation affects phosphorus homoeostasis of an aquatic herbivore. Funct. Ecol. 25, 1206–1214. doi: 10.1111/j.1365-2435.2011.01876.x
Mahowald, N., Jickells, T. D., Baker, A. R., Artaxo, P., Benitez-Nelson, C. R., Bergametti, G., et al. (2008). Global distribution of atmospheric phosphorus sources, concentrations and deposition rates, and anthropogenic impacts. Global Biogeochem. Cycles 22:GB4026. doi: 10.1029/2008GB003240
Main, T. M., Dobberfuhl, D. R., and Elser, J. J. (1997). N: P stoichiometry and ontogeny of crustacean zooplankton: a test of the growth rate hypothesis. Limnol. Oceanogr. 42, 1474–1478. doi: 10.4319/lo.1997.42.6.1474
Malzahn, A. M., Hantzsche, F., Schoo, K. L., Boersma, M., and Aberle, N. (2010). Differential effects of nutrient-limited primary production on primary, secondary or tertiary consumers. Oecologia 162, 35–48. doi: 10.1007/s00442-009-1458-y
Manzoni, S., and Porporato, A. (2011). Common hydrologic and biogeochemical controls along the soil-stream continuum. Hydrol. Process. 25, 1355–1360. doi: 10.1002/hyp.7938
Marino, R., Chan, F., Howarth, R. W., Pace, M. L., and Likens, G. E. (2006). Ecological constraints on planktonic nitrogen fixation in saline estuaries. I. nutrient and trophic controls. Mar. Ecol. Prog. Ser. 309, 25–39. doi: 10.3354/meps309025
Marleau, J. N., Guichard, F., and Loreau, M. (2015). Emergence of nutrient co-limitation through movement in stoichiometric meta-ecosystems. Ecol. Lett. 18, 1163–1173. doi: 10.1111/ele.12495
Marquet, P. A., Allen, A. P., Brown, J. H., Dunne, J. A., Enquist, B. J., Gillooly, J. F., et al. (2014). On theory in ecology. Bioscience 64, 701–710. doi: 10.1093/biosci/biu098
Martinson, H. M., Schneider, K., Gilbert, J., Hines, J. E., Hamback, P. A., and Fagan, W. F. (2008). Detritivory: stoichiometry of a neglected trophic level. Ecol. Res. 23, 487–491. doi: 10.1007/s11284-008-0471-7
McEwan, C. E. A., Gatherer, D., and McEwan, N. R. (1998). Nitrogen-fixing aerobic bacteria have higher genomic GC content than non-fixing species within the same genus. Hereditas 128, 173–178. doi: 10.1111/j.1601-5223.1998.00173.x
McGrath, J. W., and Quinn, J. P. (2000). Intracellular accumulation of polyphosphate by the yeast Candida humicola G-1 in response to acid pH. Appl. Environ. Microbiol. 66, 4068–4073. doi: 10.1128/AEM.66.9.4068-4073.2000
McGroddy, M. E., Daufresne, T., and Hedin, L. O. (2004). Scaling of C: N: P stoichiometry in forests worldwide: implications of terrestrial redfield-type ratios. Ecology 85, 2390–2401. doi: 10.1890/03-0351
Mendez, M., and Karlsson, P. S. (2005). Nutrient stoichiometry in Pinguicula vulgaris: nutrient availability, plant size, and reproductive status. Ecology 86, 982–991. doi: 10.1890/04-0354
Meunier, C. L., Boersma, M., Wiltshire, K. H., and Malzahn, A. M. (2016). Zooplankton eat what they need: copepod selective feeding and potential consequences for marine systems. Oikos 125, 50–58. doi: 10.1111/oik.02072
Meunier, C. L., Malzahn, A. M., and Boersma, M. (2014). A new approach to homeostatic regulation: towards a unified view of physiological and ecological concepts. PLoS ONE 9:e107737. doi: 10.1371/journal.pone.0107737
Mitra, A., and Flynn, K. J. (2005). Predator-prey interactions: is ‘ecological stoichiometry’ sufficient when good food goes bad? J. Plankton Res. 27, 393–399. doi: 10.1093/plankt/fbi022
Moe, S. J., Stelzer, R. S., Forman, M. R., Harpole, W. S., Daufresne, T., and Yoshida, T. (2005). Recent advances in ecological stoichiometry: insights for population and community ecology. Oikos 109, 29–39. doi: 10.1111/j.0030-1299.2005.14056.x
Monod, J. (1947). The phenomenon of enzymatic adaptation - and its bearings on problems of genetics and cellular differentiation. Growth 11, 223–289.
Morehouse, N. I., Nakazawa, T., Booher, C. M., Jeyasingh, P. D., and Hall, M. D. (2010). Sex in a material world: why the study of sexual reproduction and sex-specific traits should become more nutritionally-explicit. Oikos 119, 766–778. doi: 10.1111/j.1600-0706.2009.18569.x
Mulder, K., and Bowden, W. B. (2007). Organismal stoichiometry and the adaptive advantage of variable nutrient use and production efficiency in Daphnia. Ecol. Modell. 202, 427–440. doi: 10.1016/j.ecolmodel.2006.11.007
Muller, M., and Kersten, S. (2003). Nutrigenomics: goals and strategies. Nature Rev. Genet. 4, 315–322. doi: 10.1038/nrg1047
Nakazawa, T. (2011). The ontogenetic stoichiometric bottleneck stabilizes herbivore-autotroph dynamics. Ecol. Res. 26, 209–216. doi: 10.1007/s11284-010-0752-9
Nelson, W. A., McCauley, E., and Wrona, F. J. (2001). Multiple dynamics in a single predator-prey system: experimental effects of food quality. Proc. R. Soc. B Biol. Sci. 268, 1223–1230. doi: 10.1098/rspb.2001.1652
Okin, G. S., Mahowald, N., Chadwick, O. A., and Artaxo, P. (2004). Impact of desert dust on the biogeochemistry of phosphorus in terrestrial ecosystems. Global Biogeochem. Cycles 18:GB2005. doi: 10.1029/2003GB002145
O'Neill, R. V. (1986). A Hierarchical Concept of Ecosystems. Princeton, NJ: Princeton University Press.
Passarge, J., Hol, S., Escher, M., and Huisman, J. (2006). Competition for nutrients and light: stable coexistence, alternative stable states, or competitive exclusion? Ecol. Monogr. 76, 57–72. doi: 10.1890/04-1824
Paterson, M. J., Findlay, D. L., Salki, A. G., Hendzel, L. L., and Hesslein, R. H. (2002). The effects of Daphnia on nutrient stoichiometry and filamentous cyanobacteria: a mesocosm experiment in a eutrophic lake. Freshw. Biol. 47, 1217–1233. doi: 10.1046/j.1365-2427.2002.00842.x
Payn, R. A., Helton, A. M., Poole, G. C., Izurieta, C., Burgin, A. J., and Bernhardt, E. S. (2014). A generalized optimization model of microbially driven aquatic biogeochemistry based on thermodynamic, kinetic, and stoichiometric ecological theory. Ecol. Modell. 294, 1–18. doi: 10.1016/j.ecolmodel.2014.09.003
Peace, A. (2015). Effects of light, nutrients, and food chain length on trophic efficiencies in simple stoichiometric aquatic food chain models. Ecol. Modell. 312, 125–135. doi: 10.1016/j.ecolmodel.2015.05.019
Penn, J., Weber, T., and Deutsch, C. (2016). Microbial functional diversity alters the structure and sensitivity of oxygen deficient zones. Geophys. Res. Lett. 43, 9773–9780. doi: 10.1002/2016GL070438
Penuelas, J., Poulter, B., Sardans, J., Ciais, P., van der Velde, M., Bopp, L., et al. (2013). Human-induced nitrogen-phosphorus imbalances alter natural and managed ecosystems across the globe. Nat. Commun. 4:10. doi: 10.1038/ncomms3934
Persson, J., Fink, P., Goto, A., Hood, J. M., Jonas, J., and Kato, S. (2010). To be or not to be what you eat: regulation of stoichiometric homeostasis among autotrophs and heterotrophs. Oikos 119, 741–751. doi: 10.1111/j.1600-0706.2009.18545.x
Pickett, S. T. A., Kolasa, J., and Jones, C. G. (2007a). “2 - Understanding in Ecology,” in Ecological Understanding, 2nd Edn., eds S. T. A. Pickett, J. Kolasa and C. G. Jones (San Diego, CA: Academic Press), 33–58.
Pickett, S. T. A., Kolasa, J., and Jones, C. G. (2007b). “3 - The Anatomy of Theory,” in Ecological Understanding, 2nd Edn., eds S. T. A. Pickett, J. Kolasa and C. G. Jones (San Diego, CA: Academic Press), 61–96.
Pickett, S. T. A., Kolasa, J., and Jones, C. G. (2007c). “4 - The Ontogeny of Theory,” in Ecological Understanding 2nd Edn, eds S. T. A. Pickett, J. Kolasa and C. G. Jones (San Diego, CA: Academic Press), 97–115.
Pickett, S. T. A., Kolasa, J., and Jones, C. G. (2007d). “7 - Integration and Synthesis,” in Ecological Understanding 2nd Edn, eds S. T. A. Pickett, J. Kolasa and C. G. Jones (San Diego, CA: Academic Press), 146–167.
Porder, S., Paytan, A., and Vitousek, P. M. (2005). Erosion and landscape development affect plant nutrient status in the Hawaiian Islands. Oecologia 142, 440–449. doi: 10.1007/s00442-004-1743-8
Pulkkinen, K., Wojewodzic, M. W., and Hessen, D. O. (2014). Phosphorus limitation enhances parasite impact: feedback effects at the population level. BMC Ecol. 14:29. doi: 10.1186/s12898-014-0029-1
Ramin, M., Perhar, G., Shimoda, Y., and Arhonditsis, G. B. (2012). Examination of the effects of nutrient regeneration mechanisms on plankton dynamics using aquatic biogeochemical modeling. Ecol. Modell. 240, 139–155. doi: 10.1016/j.ecolmodel.2012.04.018
Raven, J. A. (1997). “The vacuole: a cost-benefit analysis,” in Advances in Botanical Research Incorporating Advances in Plant Pathology, Vol. 25, The Plant Vacuole, eds R. A. Leigh and D. Sanders (San Diego, CA: Elsevier Academic Press Inc), 59–86.
Redfield, A. C. (1934). “On the proportions of organic derivatives in sea water and their relation to the composition of plankton,” in James Johnstone Memorial Volume, ed R. J. Daniel (Liverpool: University Press of Liverpool).
Redfield, A. C. (1958). The biological control of chemical factors in the environment. Am. Sci. 46, 205–211.
Roos, A. M. D. E., and Persson, L. (2013). Population and Community Ecology of Ontogenetic Development. Princeton, NJ: Princeton University Press.
Ruffel, S., Krouk, G., and Coruzzi, G. M. (2010). A systems view of responses to nutritional cues in arabidopsis: toward a paradigm shift for predictive network modeling. Plant Physiol. 152, 445–452. doi: 10.1104/pp.109.148502
Salt, D. E., Baxter, I., and Lahner, B. (2008). Ionomics and the study of the plant ionome. Annu. Rev. Plant Biol. 59, 709–733. doi: 10.1146/annurev.arplant.59.032607.092942
Sardans, J., Penuelas, J., Estiarte, M., and Prieto, P. (2008). Warming and drought alter C and N concentration, allocation and accumulation in a Mediterranean shrubland. Glob. Chang. Biol. 14, 2304–2316. doi: 10.1111/j.1365-2486.2008.01656.x
Sardans, J., Rivas-Ubach, A., and Penuelas, J. (2012). The elemental stoichiometry of aquatic and terrestrial ecosystems and its relationships with organismic lifestyle and ecosystem structure and function: a review and perspectives. Biogeochemistry 111, 1–39. doi: 10.1007/s10533-011-9640-9
Schade, J. D., Espeleta, J. F., Klausmeier, C. A., McGroddy, M. E., Thomas, S. A., and Zhang, L. X. (2005). A conceptual framework for ecosystem stoichiometry: balancing resource supply and demand. Oikos 109, 40–51. doi: 10.1111/j.0030-1299.2005.14050.x
Schindler, D. W. (1977). Evolution of phosphorus limitation in lakes. Science 195, 260–262. doi: 10.1126/science.195.4275.260
Seidendorf, B., Meier, N., Petrusek, A., Boersma, M., Streit, B., and Schwenk, K. (2010). Sensitivity of Daphnia species to phosphorus-deficient diets. Oecologia 162, 349–357. doi: 10.1007/s00442-009-1452-4
Sitters, J., Atkinson, C. L., Guelzow, N., Kelly, P., and Sullivan, L. L. (2015). Spatial stoichiometry: cross-ecosystem material flows and their impact on recipient ecosystems and organisms. Oikos 124, 920–930. doi: 10.1111/oik.02392
Smith, V. H., Jones, T. P., and Smith, M. S. (2005). Host nutrition and infectious disease: an ecological view. Front. Ecol. Environ. 3, 268–274. doi: 10.1890/1540-9295(2005)003[0268:HNAIDA]2.0.CO;2
Sommer, U., Sommer, F., Feuchtmayr, H., and Hansen, T. (2004). The influence of mesozooplankton on phytoplankton nutrient limitation: a mesocosm study with northeast Atlantic plankton. Protist 155, 295–304. doi: 10.1078/1434461041844268
Steenbergh, A. K., Bodelier, P. L. E., Heldal, M., Slomp, C. P., and Laanbroek, H. J. (2013). Does microbial stoichiometry modulate eutrophication of aquatic ecosystems? Environ. Microbiol. 15, 1572–1579. doi: 10.1111/1462-2920.12042
Sterner, R., and Schulz, K. (1998). Zooplankton nutrition: recent progress and a reality check. Aquat. Ecol. 32, 261–279. doi: 10.1023/A:1009949400573
Sterner, R. W. (1986). Herbivores direct and indirect effects on algal populations. Science 231, 605–607. doi: 10.1126/science.231.4738.605
Sterner, R. W. (1990). The ratio of nitrogen to phosphorus resupplied by herbivores—zooplankton and the algal competitive arena. Am. Nat. 136, 209–229. doi: 10.1086/285092
Sterner, R. W. (1998). Demography of a natural population of Daphnia retrocurva in a lake with low food quality. J. Plankton Res. 20, 471–489. doi: 10.1093/plankt/20.3.471
Sterner, R. W. (2008). On the phosphorus limitation paradigm for lakes. Int. Rev. Hydrobiol. 93, 433–445. doi: 10.1002/iroh.200811068
Sterner, R. W., Andersen, T., Elser, J. J., Hessen, D. O., Hood, J. M., McCauley, E., et al. (2008). Scale-dependent carbon: nitrogen: phosphorus seston stoichiometry in marine and freshwaters. Limnol. Oceanogr. 53, 1169–1180. doi: 10.4319/lo.2008.53.3.1169
Sterner, R. W., and Elser, J. J. (2002). Ecological Stoichiometry: The Biology of Elements from Molecules to the Biosphere. Princeton, NJ: Princeton University Press.
Sterner, R. W., Elser, J. J., Fee, E. J., Guildford, S. J., and Chrzanowski, T. H. (1997). The light:nutrient ratio in lakes: the balance of energy and materials affects ecosystem structure and process. Am. Nat. 150, 663–684. doi: 10.1086/286088
Sterner, R. W., Elser, J. J., and Hessen, D. O. (1992). Stoichiometric relationships among producers, consumers and nutrient cycling in pelagic ecosystems. Biogeochemistry 17, 49–67. doi: 10.1007/BF00002759
Sterner, R. W., and George, N. B. (2000). Carbon, nitrogen, and phosphorus stoichiometry of cyprinid fishes. Ecology 81, 127–140. doi: 10.1890/0012-9658(2000)081[0127:CNAPSO]2.0.CO;2
Sterner, R. W., Hagemeier, D. D., and Smith, W. L. (1993). Phytoplankton nutrient limitation and food quality for Daphnia. Limnol. Oceanogr. 38, 857–871. doi: 10.4319/lo.1993.38.4.0857
Sterner, R. W., and Hessen, D. O. (1994). Algal nutrient limitation and the nutrition of aquatic herbivores. Annu. Rev. Ecol. Syst. 25, 1–29. doi: 10.1146/annurev.es.25.110194.000245
Sterner, R. W., and Schwalbach, M. S. (2001). Diel integration of food quality by Daphnia: luxury consumption by a freshwater planktonic herbivore. Limnol. Oceanogr. 46, 410–416. doi: 10.4319/lo.2001.46.2.0410
Stramma, L., Schmidtko, S., Levin, L. A., and Johnson, G. C. (2010). Ocean oxygen minima expansions and their biological impacts. Deep Sea Res. Oceanog. Res. Pap. 57, 587–595. doi: 10.1016/j.dsr.2010.01.005
Striebel, M., Behl, S., and Stibor, H. (2009). The coupling of biodiversity and productivity in phytoplankton communities: consequences for biomass stoichiometry. Ecology 90, 2025–2031. doi: 10.1890/08-1409.1
Sutherland, W. J., Freckleton, R. P., Godfray, H. C. J., Beissinger, S. R., Benton, T., Cameron, D. D., et al. (2013). Identification of 100 fundamental ecological questions. J. Ecol. 101, 58–67. doi: 10.1111/1365-2745.12025
Tadonleke, R. D., Pollet, T., Van Rijswijk, P., Leberre, B., and Middelburg, J. J. (2016). Effects of oxygen loss on carbon processing and heterotrophic prokaryotes from an estuarine ecosystem: results from stable isotope probing and cytometry analyses. Estuar. Coasts 39, 992–1005. doi: 10.1007/s12237-015-0053-1
Thornton, P. E., Doney, S. C., Lindsay, K., Moore, J. K., Mahowald, N., Randerson, J. T., et al. (2009). Carbon-nitrogen interactions regulate climate-carbon cycle feedbacks: results from an atmosphere-ocean general circulation model. Biogeosciences 6, 2099–2120. doi: 10.5194/bg-6-2099-2009
Tilman, D. (1982). Resource competition and community structure. Princeton, NJ: Princeton University Press.
Tuljapurkar, S., and Caswell, H. (1997). Structured-Population Models in Marine, Terrestrial, and Freshwater Systems. New York, NY: Springer.
Urabe, J., and Sterner, R. W. (2001). Contrasting effects of different types of resource depletion on life-history traits in Daphnia. Funct. Ecol. 15, 165–174. doi: 10.1046/j.1365-2435.2001.00511.x
van Groenigen, K. J., Six, J., Hungate, B. A., de Graaff, M. A., van Breemen, N., and van Kessel, C. (2006). Element interactions limit soil carbon storage. Proc. Natl. Acad. Sci. U.S.A. 103, 6571–6574. doi: 10.1073/pnas.0509038103
Vanni, M. J., Boros, G., and McIntyre, P. B. (2013). When are fish sources vs. sinks of nutrients in lake ecosystems? Ecology 94, 2195–2206. doi: 10.1890/12-1559.1
Vanni, M. J., Flecker, A. S., Hood, J. M., and Headworth, J. L. (2002). Stoichiometry of nutrient recycling by vertebrates in a tropical stream: linking species identity and ecosystem processes. Ecol. Lett. 5, 285–293. doi: 10.1046/j.1461-0248.2002.00314.x
Vanni, M. J., and McIntyre, P. B. (2016). Predicting nutrient excretion of aquatic animals with metabolic ecology and ecological stoichiometry: a global synthesis. Ecology 97, 3460–3471. doi: 10.1002/ecy.1582
Vieira-Silva, S., Touchon, M., and Rocha, E. P. C. (2010). No evidence for elemental-based streamlining of prokaryotic genomes. Trends Ecol. Evol. 25, 319–320. doi: 10.1016/j.tree.2010.03.001
Vitousek, P. M., and Farrington, H. (1997). Nutrient limitation and soil development: experimental test of a biogeochemical theory. Biogeochemistry 37, 63–75. doi: 10.1023/A:1005757218475
Vrede, T., Dobberfuhl, D. R., Kooijman, S., and Elser, J. J. (2004). Fundamental connections among organism C: N: P stoichiometry, macromolecular composition, and growth. Ecology 85, 1217–1229. doi: 10.1890/02-0249
Wagner, N. D., Hillebrand, H., Wacker, A., and Frost, P. C. (2013). Nutritional indicators and their uses in ecology. Ecol. Lett. 16, 535–544. doi: 10.1111/ele.12067
Wang, H., Sterner, R. W., and Elser, J. J. (2012). On the “strict homeostasis” assumption in ecological stoichiometry. Ecol. Modell. 243, 81–88. doi: 10.1016/j.ecolmodel.2012.06.003
Wang, Y. P., Law, R. M., and Pak, B. (2010). A global model of carbon, nitrogen and phosphorus cycles for the terrestrial biosphere. Biogeosciences 7, 2261–2282. doi: 10.5194/bg-7-2261-2010
Wassen, M. J., de Boer, H. J., Fleischer, K., Rebel, K. T., and Dekker, S. C. (2013). Vegetation-mediated feedback in water, carbon, nitrogen and phosphorus cycles. Landsc. Ecol. 28, 599–614. doi: 10.1007/s10980-012-9843-z
Weber, T. S., and Deutsch, C. (2010). Ocean nutrient ratios governed by plankton biogeography. Nature 467, 550–554. doi: 10.1038/nature09403
Weider, L. J., Elser, J. J., Crease, T. J., Mateos, M., Cotner, J. B., and Markow, T. A. (2005). The functional significance of ribosomal (r)DNA variation: impacts on the evolutionary ecology of organisms. Ann. Rev. Ecol. Evol. Syst. 36, 219–242. doi: 10.1146/annurev.ecolsys.36.102003.152620
Woods, H. A., Fagan, W. F., Elser, J. J., and Harrison, J. F. (2004). Allometric and phylogenetic variation in insect phosphorus content. Funct. Ecol. 18, 103–109. doi: 10.1111/j.1365-2435.2004.00823.x
Worman, C. O., and Kimbrell, T. (2008). Getting to the hart of the matter: did antlers truly cause the extinction of the Irish elk? Oikos 117, 1397–1405. doi: 10.1111/j.0030-1299.2008.16608.x
Wunch, D., Toon, G. C., Blavier, J. F. L., Washenfelder, R. A., Notholt, J., Connor, B. J., et al. (2011). The total carbon column observing network. Philos. Trans. R. Soc. Math. Phys. Eng. Sci. 369, 2087–2112. doi: 10.1098/rsta.2010.0240
Xia, C. X., Yu, D., Wang, Z., and Xie, D. (2014). Stoichiometry patterns of leaf carbon, nitrogen and phosphorous in aquatic macrophytes in eastern China. Ecol. Eng. 70, 406–413. doi: 10.1016/j.ecoleng.2014.06.018
Yamamichi, M., Meunier, C. L., Peace, A., Prater, C., and Rua, M. A. (2015). Rapid evolution of a consumer stoichiometric trait destabilizes consumer-producer dynamics. Oikos 124, 960–969. doi: 10.1111/oik.02388
Keywords: biological organization, biological stoichiometry, consumer-driven nutrient recycling, ecological theory, theory integration, growth-rate hypothesis, light:nutrient hypothesis
Citation: Cherif M, Faithfull C, Guo J, Meunier CL, Sitters J, Uszko W and Rivera Vasconcelos F (2017) An Operational Framework for the Advancement of a Molecule-to-Biosphere Stoichiometry Theory. Front. Mar. Sci. 4:286. doi: 10.3389/fmars.2017.00286
Received: 30 November 2016; Accepted: 22 August 2017;
Published: 08 September 2017.
Edited by:
James Cotner, University of Minnesota, United StatesReviewed by:
Ursula Felicitas Marianne Witte, University of Aberdeen, United KingdomAnna Villnäs, University of Helsinki, Finland
Priscilla Licandro, Plymouth Marine Laboratory, United Kingdom
Copyright © 2017 Cherif, Faithfull, Guo, Meunier, Sitters, Uszko and Rivera Vasconcelos. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mehdi Cherif, mehdi.cherif@umu.se
 Mehdi Cherif
Mehdi Cherif Carolyn Faithfull1
Carolyn Faithfull1  Cédric L. Meunier
Cédric L. Meunier Judith Sitters
Judith Sitters Wojciech Uszko
Wojciech Uszko Francisco Rivera Vasconcelos
Francisco Rivera Vasconcelos