Crystal Chemistry of an Erythrite-Köttigite Solid Solution (Co3–xZnx) (AsO4)2·8H2O
Abstract
:1. Introduction
2. Sampling Site
3. Experimental Methods
3.1. Sample Description
3.2. Scanning Electron Microscopy (SEM)
3.3. Electron Probe Microanalysis (EPMA)
3.4. Structure Refinement
3.5. X-ray Powder Diffraction (XRD)
3.6. Raman Spectroscopy
4. Results and Discussion
4.1. Paragenetic Sequence
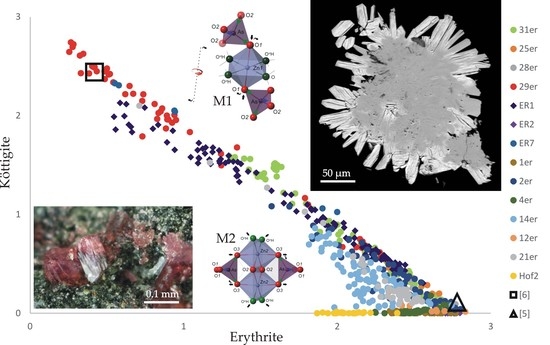
4.2. Chemical Composition
4.3. Structure Refinement
4.4. Unit-Cell Dimensions
4.5. Raman Spectroscopy
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wildner, M.; Giester, G.; Lengauer, C.; McCammon, C. Structure and crystal chemistry of vivianite-type compounds: Crystal structures of erythrite and annabergite with a Mössbauer study of erythrite. Eur. J. Miner. 1996, 8, 187–192. [Google Scholar] [CrossRef]
- Giuseppetti, G.; Tadini, C. The crystal structure of cabrerite, (Ni, Mg)3(AsO4)2·H2O, a variety of annabergite. Bull. Minéral. 1982, 105, 333–337. [Google Scholar] [CrossRef]
- Plášil, J.; Škácha, P.; Sejkora, J.; Škoda, R.; Novák, M.; Veselovský, F.; Hloušek, J. Babánekite, Cu3(AsO4)2 ∙8H2O, from Jáchymov, Czech Republic—A new member of the vivianite group. J. Geosci. 2017, 62, 261–270. [Google Scholar] [CrossRef] [Green Version]
- Capitelli, F.; Elaatmani, M.; Lalaoui, M.D.; Piniella, J.F. Crystal structure of a vivianite-type mineral: Mg-rich erythrite, (Co2.16Ni0.24Mg0.60)(AsO4)2·8H2O. Zeitschrift für Kristallographie -Cryst. Mater. 2007, 222, 676–679. [Google Scholar] [CrossRef]
- Antao, S.M.; Dhaliwal, I. Growth oscillatory zoning in erythrite, ideally Co3(AsO4)2·8H2O: Structural variations in vivianite-group minerals. Minerals 2017, 7, 136. [Google Scholar] [CrossRef]
- Hill, R.J. The crystal structure of köttigite. Am. Mineral. 1979, 64, 376–382. [Google Scholar]
- Yoshiasa, A.; Miyano, Y.; Isobe, H.; Sugiyama, K.; Arima, H.; Nakatsuka, A.; Momma, K.; Miyawaki, R. Structural refinement of köttigite–parasymplesite solid solution: Unique cation site occupancy and chemical bonding with water molecules. J. Miner. Pet. Sci. 2016, 111, 363–369. [Google Scholar] [CrossRef] [Green Version]
- Mori, H.; Ito, T. The structure of vivianite and symplesite. Acta Crystallogr. 1950, 3, 1–6. [Google Scholar] [CrossRef]
- Markl, G.; Marks, M.A.W.; Derrey, I.; Gühring, J.-E. Weathering of cobalt arsenides: Natural assemblages and calculated stability relations among secondary Ca-Mg-Co arsenates and carbonates. Am. Miner. 2014, 99, 44–56. [Google Scholar] [CrossRef]
- Dumańska-Słowik, M.; Pieczka, A.; Natkaniec-Nowak, L.; Kunecki, P.; Gaweł, A.; Heflik, W.; Smoliński, W.; Kozub-Budzyń, G. Mg-enriched erythrite from Bou Azzer, Anti-Atlas Mountains, Morocco: Geochemical and spectroscopic characteristics. Miner. Pet. 2018, 112, 381–392. [Google Scholar] [CrossRef] [Green Version]
- Siuda, R.; Macioch, A. Secondary arsenic minerals from the Złoty Stok As-Au abandoned mine (SW Poland). Geol. Quaterly 2018, 62, 925–940. [Google Scholar] [CrossRef] [Green Version]
- Anthony, J.W.; Bideaux, R.A.; Bladh, K.W.; Nichols, M.C. Handbook of Mineralogy: Arsenates, Phosphates, Vanadates; Mineral Data Publishing: Tucson, AZ, USA, 2000; Volume IV, p. 159. [Google Scholar]
- Jambor, J.L.; Dutrizac, J.E. Solid solutions in the annabergite-erythrite-hörnesite synthetic system. Can. Miner. 1995, 33, 1063–1071. [Google Scholar]
- Martens, W.N.; Kloprogge, J.T.; Frost, R.L.; Rintoul, L. Site occupancy of Co and Ni in erythrite-annabergite solid solutions deduced by vibrational spectroscopy. Can. Miner. 2005, 43, 1065–1075. [Google Scholar] [CrossRef]
- Wei, C.; Zhu, Y.; Zhang, X.; Wang, X.; Liu, J. Dissolution and solubility of the erythrite/annabergite solid solution [(CoxNi1-x)3(AsO4)2·8H2O] at 25 °C. Asian J. Chem. 2013, 25, 7687–7696. [Google Scholar] [CrossRef]
- Sturman, B.D. New data for köttigite and parasymplesite. Can. Miner. 1976, 14, 437–441. [Google Scholar]
- Shannon, R.D. Revised effective ionic radii and systematic studies if interatomic distances in halides and chalcogenides. Acta Cryst. 1976, A32, 751–767. [Google Scholar] [CrossRef]
- Ciesielczuk, J.; Janeczek, J.; Dulski, M.; Krzykawski, T. Pseudomalachite–cornwallite and kipushite–philipsburgite solid solutions: Chemical composition and Raman spectroscopy. Eur. J. Miner. 2016, 28, 555–569. [Google Scholar] [CrossRef]
- Mochnacka, K.; Oberc-Dziedzic, T.; Mayer, W.; Pieczka, A. Ore mineralization related to geological evolution of the Karkonosze-Izera Massif (the Sudetes, Poland)–Towards a model. Ore Geol. Rev. 2015, 64, 215–238. [Google Scholar] [CrossRef]
- Parafiniuk, J.; Siuda, R.; Borkowski, A. Sulphate and arsenate minerals as environmental indicators in the weathering zones of selected ore deposits, Western Sudetes, Poland. Acta Geol. Pol. 2016, 66, 493–508. [Google Scholar] [CrossRef]
- Siuda, R.; Gołębiowska, B. New data on supergene minerals from Miedzianka-Ciechanowice deposit in the Rudawy Janowickie Mountains (Lower Silesia, Poland). Prz. Geol. 2011, 59, 226–234, (In Polish with English Abstract). [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, 71, 3–8. [Google Scholar]
- Lee, J.S.; Nriagu, J.O. Stability constants for metal arsenates. Environ. Chem. 2007, 4, 123–133. [Google Scholar] [CrossRef]
- Charykova, M.V.; Krivovichev, O.S.; Yakovenko, O.S.; Depmeier, W. Thermodynamics of arsenates, selenites, and sulfates in the oxidation zone of sulfide ores: Part III: Eh–pH diagrams of the Me–As–H2O Systems (Me = Co, Ni, Fe, Cu, Zn, Pb) at 25°C. Geol. Ore Depos. 2011, 53, 501–513. [Google Scholar] [CrossRef]
- Magalhães, M.C.F.; Pedrosa de Jesus, J.D. The chemistry of formation of some secondary arsenate minerals of Cu(II), Zn(II) and Pb(II). Miner. Mag. 1988, 52, 679–690. [Google Scholar] [CrossRef] [Green Version]
- Frost, R.L.; Martens, W.N.; Williams, P.; Kloprogge, J.T. Raman spectroscopic study of the vivianite arsenate minerals. J. Raman Spectrosc. 2003, 34, 751–759. [Google Scholar] [CrossRef] [Green Version]
- Rojo, J.M.; Mesa, J.L.; Pizarro, J.L.; Lezama, L.; Arriotua, M.I.; Rojo, T. Spectroscopic and magnetic study of the (Mg,M)3(AsO4)2 8H2O (M = Ni2+, Co2+) arsenates. Mater. Res. Bull. 1996, 31, 925–934. [Google Scholar] [CrossRef]
- Frost, R.L.; Kloprogge, T.; Weier, M.L.; Martens, W.N.; Ding, Z.; Edwards, G.H. Raman spectroscopy of selected arsenates–implications for soil remediation. Spectrochim. Acta A 2003, 59, 2241–2246. [Google Scholar] [CrossRef] [Green Version]
- Martens, W.N.; Frost, R.L.; Kloprogge, T. Raman spectroscopy of synthetic erythrite, partially dehydrated erythrite and hydrothermally synthesized dehydrated erythrite. J. Raman Spectrosc. 2003, 34, 90–95. [Google Scholar] [CrossRef] [Green Version]
- Martens, W.N.; Kloprogge, T.; Frost, R.L.; Rintoul, L. Single-crystal Raman study of erythrite Co3(AsO4)2·H2O. J. Raman Spectrosc. 2004, 35, 208–216. [Google Scholar] [CrossRef] [Green Version]
- Makreski, P.; Stefov, S.; Pejov, L.; Jovanovski, G. Theoretical and experimental study of the vibrational spectra of (para)symplesite and hornesite. Spectrochim. Acta A 2015, 144, 155–162. [Google Scholar] [CrossRef]
- Čejka, J.; Sejkora, J.; Bahfenne, S.; Palmer, S.J.; Plášil, J.; Frost, R.L. Raman spectroscopy of hydrogen-arsenate group (AsO3OH) in solid-state compounds: Cobalt mineral phase burgessite Co2(H2O)[AsO3OH]2·H2O. J. Raman Spectrosc. 2011, 42, 214–218. [Google Scholar] [CrossRef] [Green Version]
- Libowitzky, E. Correlation of the O-H stretching frequencies and the OH… H hydrogen bond lengths in minerals. Monatschefte für Chemie 1999, 130, 1047–1049. [Google Scholar]













| Erythrite Co3(AsO4)2·8H2O | Annabergite Ni3(AsO4)2·8H2O | Köttigite Zn3(AsO4)2·8H2O | Hörnesite Mg3(AsO4)2·8H2O | Parasymplesite Fe3(AsO4)2·8H2O | Ref. |
|---|---|---|---|---|---|
| 93 | 2 | 4 | - | 1 | [5] |
| 89 | - | - | - | 11 | [12] |
| 84 | 3 | - | 11 | 2 | [10] |
| 81 | 5 | - | 12 | 2 | [10] |
| 75 | 5 | 1 | 19 | 1 | [10] |
| 72 | 8 | - | 20 | - | [4] |
| 71 | 5 | 1 | 22 | 1 | [10] |
| 67 | 8 | - | - | 25 | [1] |
| 67 | 10 | - | 20 | - | [11] # |
| 50 | 5 | - | 45 | - | [10] |
| 49 | 5 | - | 46 | - | [10] |
| 46 | 8 | - | 42 | - | [11] # |
| 22 | 43 | - | 30 | - | [11] # |
| 19 | 51 | - | 27 | - | [11] |
| 19 | 5 | 76 | - | - | [16] |
| 14 | 5 | 81 | - | - | [16] |
| - | 83 | - | 17 | - | [1] |
| - | 74 | - | 23 | 3 | [2] |
| - | - | 46 | - | 54 | [7] |
| - | - | 44 | - | 56 | [7] |
| Crystal Data | |
|---|---|
| Chemical formula | As4Co2.16H32O32Zn3.84 |
| Mr | 1222.24 |
| Crystal system, space group | Monoclinic, C2/m |
| Temperature (K) | 293 |
| a, b, c (Å) | 10.2588 (3), 13.4200 (4), 4.76200 (14) |
| β (°) | 105.232 (3) |
| V (Å3) | 632.56 (3) |
| Z | 1 |
| F(000) | 594 |
| Radiation type | Mo Kα |
| µ (mm−1) | 10.29 |
| Crystal size (mm) | 0.18 × 0.09 × 0.07 |
| Data Collection | |
| Absorption correction | Multi-scan CrysAlis PRO 1.171.38.41q (Rigaku Oxford Diffraction, 2015) Empirical absorption correction using spherical harmonics, implemented in SCALE3 ABSPACK scaling algorithm. |
| Tmin, Tmax | 0.528, 1.000 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 2474, 676, 661 |
| Rint | 0.014 |
| Θ values (°) | Θmax = 26.4, Θmin = 3.0 |
| (sin Θ/λ)max (Å−1) | 0.625 |
| Range of h, k, l | h = −12 → 12, k = −16 → 12, l = −5 → 5 |
| Refinement | |
| R[F2 > 2σ(F2)], wR(F2), S | 0.021, 0.060, 1.15 |
| No. of reflections | 676 |
| No. of parameters | 55 |
| No. of restraints | 6 |
| H-atom treatment | H-atom parameters constrained |
| Δ > max, Δ > min (e Å−3) | 0.77, −0.75 |
| Analysis Number | CoO | ZnO | MgO | NiO | FeO | As2O5 | P2O5 | SO2 | CaO | SiO2 | Total | Co | Zn | Mg | Ni | Fe | Mn | ΣX | As | P | ΣA |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E | 37.54 | - | - | - | - | 38.39 | - | - | - | - | 75.93 | 3.00 | - | - | - | - | - | 3.00 | 2.00 | - | 2.00 |
| 1 | 34.17 | 0.73 | 0.87 | 0.41 | 0.13 | 37.86 | 0.02 | 0.01 | 0.21 | 0.05 | 74.46 | 2.77 | 0.05 | 0.13 | 0.03 | 0.01 | 0.00 | 3.00 | 2.00 | 0.00 | 2.00 |
| 2 | 33.85 | 1.19 | 0.75 | 1.12 | 0.00 | 38.75 | 0.09 | 0.05 | 0.37 | 0.06 | 76.22 | 2.69 | 0.09 | 0.11 | 0.09 | 0.00 | 0.00 | 2.98 | 2.01 | 0.01 | 2.02 |
| 3 | 33.36 | 1.71 | 0.82 | 0.63 | 0.11 | 38.49 | 0.00 | 0.00 | 0.25 | 0.01 | 75.50 | 2.67 | 0.13 | 0.12 | 0.05 | 0.01 | 0.01 | 2.99 | 2.01 | 0.00 | 2.01 |
| 4 | 32.58 | 0.62 | 1.24 | 1.10 | 0.07 | 37.72 | 0.03 | 0.10 | 0.25 | 0.02 | 73.75 | 2.66 | 0.05 | 0.19 | 0.09 | 0.01 | 0.00 | 2.99 | 2.01 | 0.00 | 2.01 |
| 5 | 31.77 | 2.35 | 0.60 | 1.06 | 0.05 | 37.79 | 0.00 | 0.16 | 0.13 | 0.27 | 74.22 | 2.61 | 0.18 | 0.09 | 0.09 | 0.00 | 0.00 | 2.98 | 2.02 | 0.00 | 2.02 |
| 6 | 31.72 | 0.22 | 1.05 | 2.00 | 0.27 | 37.66 | 0.06 | 0.27 | 0.64 | 0.23 | 74.19 | 2.61 | 0.02 | 0.16 | 0.16 | 0.02 | 0.01 | 2.98 | 2.02 | 0.01 | 2.02 |
| 7 | 31.44 | 2.54 | 1.03 | 0.41 | 0.19 | 37.42 | 0.00 | 0.21 | 0.47 | 0.07 | 73.85 | 2.59 | 0.19 | 0.16 | 0.03 | 0.02 | 0.01 | 2.99 | 2.01 | 0.00 | 2.01 |
| 8 | 30.23 | 1.53 | 1.25 | 1.20 | 0.03 | 36.75 | 0.02 | 0.05 | 0.46 | 0.45 | 72.01 | 2.55 | 0.12 | 0.20 | 0.10 | 0.00 | 0.00 | 2.98 | 2.02 | 0.00 | 2.02 |
| 9 | 30.79 | 0.11 | 1.74 | 1.39 | 0.10 | 37.14 | 0.00 | 0.19 | 0.52 | 0.02 | 72.74 | 2.54 | 0.01 | 0.27 | 0.12 | 0.01 | 0.06 | 3.00 | 2.00 | 0.00 | 2.00 |
| 10 | 30.81 | 0.38 | 2.08 | 1.51 | 0.05 | 37.12 | 0.04 | 0.13 | 0.57 | 0.01 | 72.80 | 2.53 | 0.03 | 0.32 | 0.12 | 0.00 | 0.01 | 3.01 | 1.99 | 0.00 | 1.99 |
| 11 | 30.14 | 2.00 | 1.27 | 1.07 | 0.00 | 36.80 | 0.00 | 0.01 | 0.59 | 0.00 | 72.11 | 2.53 | 0.15 | 0.20 | 0.09 | 0.00 | 0.02 | 2.99 | 2.01 | 0.00 | 2.01 |
| 12 | 30.59 | 0.00 | 1.58 | 2.00 | 0.28 | 37.04 | 0.00 | 0.08 | 0.82 | 0.26 | 73.30 | 2.52 | 0.00 | 0.24 | 0.17 | 0.02 | 0.06 | 3.01 | 1.99 | 0.00 | 1.99 |
| 13 | 29.83 | 4.61 | 1.11 | 0.24 | 0.18 | 37.08 | 0.00 | 0.21 | 0.19 | 0.15 | 73.59 | 2.46 | 0.35 | 0.17 | 0.02 | 0.02 | 0.00 | 3.01 | 1.99 | 0.00 | 1.99 |
| 14 | 29.83 | 3.21 | 1.25 | 0.96 | 0.19 | 37.95 | 0.01 | 0.12 | 0.63 | 0.11 | 74.51 | 2.43 | 0.24 | 0.19 | 0.08 | 0.02 | 0.02 | 2.98 | 2.02 | 0.00 | 2.02 |
| 15 | 29.62 | 0.00 | 2.59 | 1.57 | 0.10 | 37.59 | 0.04 | 0.25 | 0.70 | 0.00 | 72.70 | 2.43 | 0.00 | 0.39 | 0.13 | 0.01 | 0.02 | 2.98 | 2.01 | 0.00 | 2.02 |
| 16 | 28.48 | 5.15 | 1.49 | 0.46 | 0.17 | 38.26 | 0.00 | 0.04 | 0.43 | 0.13 | 74.72 | 2.31 | 0.38 | 0.22 | 0.04 | 0.01 | 0.01 | 2.98 | 2.02 | 0.00 | 2.02 |
| 17 | 28.05 | 8.25 | 0.37 | 0.31 | 0.00 | 37.84 | 0.20 | 0.04 | 0.58 | 0.05 | 75.69 | 2.27 | 0.62 | 0.06 | 0.04 | 0.00 | 0.00 | 2.98 | 2.00 | 0.02 | 2.02 |
| 18 | 27.68 | 7.94 | 0.91 | 0.44 | 0.05 | 37.57 | 0.06 | 0.02 | 0.38 | 0.40 | 75.45 | 2.24 | 0.59 | 0.14 | 0.04 | 0.00 | 0.00 | 3.01 | 1.98 | 0.01 | 1.99 |
| 19 | 26.61 | 5.37 | 1.36 | 0.55 | 0.75 | 36.83 | 0.01 | 0.06 | 0.12 | 0.03 | 71.72 | 2.24 | 0.42 | 0.21 | 0.05 | 0.07 | 0.00 | 2.98 | 2.02 | 0.00 | 2.02 |
| 20 | 27.66 | 1.70 | 3.67 | 0.99 | 0.24 | 37.59 | 0.00 | 0.03 | 0.53 | 0.00 | 72.60 | 2.23 | 0.13 | 0.55 | 0.08 | 0.02 | 0.02 | 3.02 | 1.98 | 0.00 | 1.98 |
| 21 | 26.64 | 4.44 | 2.68 | 0.40 | 0.25 | 37.22 | 0.00 | 0.13 | 0.25 | 0.76 | 72.98 | 2.19 | 0.34 | 0.41 | 0.03 | 0.02 | 0.02 | 3.01 | 1.99 | 0.00 | 1.99 |
| 22 | 28.24 | 9.94 | 0.46 | 0.78 | 0.00 | 39.45 | 0.20 | 0.03 | 0.38 | 0.20 | 79.74 | 2.18 | 0.71 | 0.07 | 0.03 | 0.00 | 0.01 | 2.99 | 1.99 | 0.02 | 2.01 |
| 23 | 27.44 | 2.90 | 3.56 | 0.70 | 0.32 | 38.46 | 0.00 | 0.08 | 0.28 | 0.09 | 74.10 | 2.17 | 0.21 | 0.52 | 0.06 | 0.03 | 0.02 | 3.01 | 1.99 | 0.00 | 1.99 |
| 24 | 27.29 | 9.54 | 0.33 | 0.49 | 0.07 | 38.45 | 0.22 | 0.06 | 0.37 | 0.08 | 77.04 | 2.17 | 0.70 | 0.05 | 0.05 | 0.01 | 0.01 | 2.99 | 1.99 | 0.02 | 2.01 |
| 25 | 26.54 | 9.19 | 0.34 | 0.64 | 0.38 | 37.18 | 0.10 | 0.09 | 0.26 | 0.38 | 75.14 | 2.17 | 0.69 | 0.05 | 0.06 | 0.03 | 0.00 | 3.01 | 1.98 | 0.01 | 1.99 |
| 26 | 26.11 | 9.41 | 0.49 | 0.46 | 0.14 | 37.03 | 0.00 | 0.06 | 0.30 | 0.17 | 74.17 | 2.16 | 0.72 | 0.07 | 0.04 | 0.01 | 0.00 | 3.00 | 2.00 | 0.00 | 2.00 |
| 27 | 27.74 | 2.32 | 4.04 | 0.88 | 0.05 | 39.69 | 0.02 | 0.16 | 0.27 | 0.00 | 75.38 | 2.15 | 0.17 | 0.58 | 0.07 | 0.00 | 0.02 | 2.99 | 2.01 | 0.00 | 2.01 |
| 28 | 27.42 | 6.94 | 2.09 | 0.58 | 0.35 | 40.19 | 0.01 | 0.06 | 0.25 | 0.19 | 78.21 | 2.11 | 0.49 | 0.30 | 0.04 | 0.03 | 0.01 | 2.98 | 2.02 | 0.00 | 2.02 |
| 29 | 25.64 | 6.79 | 1.70 | 0.86 | 0.32 | 37.41 | 0.00 | 0.11 | 0.32 | 0.04 | 73.40 | 2.11 | 0.51 | 0.26 | 0.07 | 0.03 | 0.02 | 3.00 | 2.00 | 0.00 | 2.00 |
| 30 | 24.88 | 6.15 | 3.00 | 0.53 | 0.14 | 37.33 | 0.03 | 0.00 | 0.25 | 2.20 | 74.72 | 2.03 | 0.46 | 0.45 | 0.04 | 0.01 | 0.02 | 3.02 | 1.98 | 0.00 | 1.98 |
| 31 | 25.46 | 13.26 | 0.36 | 0.50 | 0.06 | 39.42 | 0.04 | 0.28 | 0.64 | 0.21 | 80.35 | 1.96 | 0.94 | 0.05 | 0.04 | 0.00 | 0.01 | 3.01 | 1.98 | 0.00 | 1.99 |
| 32 | 23.66 | 12.93 | 0.38 | 0.37 | 0.35 | 37.41 | 0.11 | 0.06 | 0.29 | 0.35 | 75.99 | 1.92 | 0.97 | 0.06 | 0.03 | 0.03 | 0.01 | 3.01 | 1.98 | 0.01 | 1.99 |
| 33 | 24.04 | 12.52 | 0.53 | 0.70 | 0.09 | 39.09 | 0.00 | 0.61 | 0.18 | 0.14 | 78.01 | 1.91 | 0.92 | 0.08 | 0.06 | 0.01 | 0.01 | 2.98 | 2.02 | 0.00 | 2.02 |
| 34 | 17.18 | 18.19 | 0.15 | 0.47 | 0.09 | 36.13 | 0.00 | 0.16 | 0.08 | 0.08 | 72.53 | 1.47 | 1.44 | 0.02 | 0.04 | 0.01 | 0.00 | 2.98 | 2.02 | 0.00 | 2.02 |
| 35 | 16.10 | 22.50 | 0.23 | 0.06 | 0.12 | 38.82 | 0.03 | 0.00 | 0.09 | 0.09 | 78.03 | 1.27 | 1.64 | 0.03 | 0.04 | 0.01 | 0.00 | 3.00 | 2.00 | 0.00 | 2.00 |
| 36 | 15.92 | 25.63 | 0.11 | 0.25 | 0.45 | 41.89 | 0.05 | 0.35 | 0.12 | 0.18 | 84.94 | 1.17 | 1.73 | 0.01 | 0.03 | 0.03 | 0.00 | 2.99 | 2.01 | 0.00 | 2.01 |
| 37 | 12.60 | 25.21 | 0.36 | 0.19 | 0.05 | 38.45 | 0.07 | 0.00 | 0.10 | 0.14 | 77.17 | 1.01 | 1.87 | 0.05 | 0.04 | 0.00 | 0.00 | 2.98 | 2.02 | 0.01 | 2.02 |
| 38 | 12.31 | 30.00 | 0.17 | 0.14 | 0.25 | 41.70 | 0.06 | 0.01 | 0.02 | 0.16 | 84.84 | 0.91 | 2.03 | 0.02 | 0.01 | 0.02 | 0.00 | 2.99 | 2.00 | 0.00 | 2.01 |
| 39 | 11.76 | 32.19 | 0.23 | 0.13 | 0.42 | 43.68 | 0.00 | 0.00 | 0.11 | 0.20 | 88.72 | 0.83 | 2.09 | 0.03 | 0.01 | 0.03 | 0.00 | 2.99 | 2.01 | 0.00 | 2.01 |
| 40 | 11.21 | 32.42 | 0.12 | 0.29 | 0.36 | 43.53 | 0.00 | 0.21 | 0.08 | 0.25 | 88.48 | 0.80 | 2.12 | 0.02 | 0.02 | 0.03 | 0.00 | 2.98 | 2.02 | 0.00 | 2.02 |
| 41 | 11.22 | 33.86 | 0.22 | 0.14 | 0.07 | 43.34 | 0.11 | 0.12 | 0.09 | 0.46 | 89.62 | 0.79 | 2.18 | 0.03 | 0.01 | 0.01 | 0.00 | 3.01 | 1.98 | 0.01 | 1.99 |
| 42 | 10.15 | 32.03 | 0.19 | 0.08 | 0.08 | 41.21 | 0.11 | 0.21 | 0.15 | 1.00 | 85.31 | 0.76 | 2.19 | 0.03 | 0.00 | 0.01 | 0.01 | 2.99 | 2.00 | 0.01 | 2.01 |
| 43 | 9.94 | 33.69 | 0.27 | 0.48 | 0.09 | 42.83 | 0.03 | 0.00 | 0.00 | 0.40 | 87.73 | 0.71 | 2.22 | 0.04 | 0.03 | 0.01 | 0.00 | 3.00 | 1.99 | 0.00 | 2.00 |
| 44 | 8.18 | 36.04 | 0.24 | 0.16 | 0.45 | 43.88 | 0.03 | 0.15 | 0.00 | 0.15 | 89.28 | 0.58 | 2.33 | 0.03 | 0.01 | 0.03 | 0.00 | 2.99 | 2.01 | 0.00 | 2.01 |
| 45 | 7.21 | 36.62 | 0.23 | 0.18 | 0.03 | 42.60 | 0.00 | 0.04 | 0.00 | 0.31 | 87.23 | 0.52 | 2.43 | 0.03 | 0.01 | 0.00 | 0.00 | 3.00 | 2.00 | 0.00 | 2.00 |
| 46 | 7.03 | 37.11 | 0.37 | 0.00 | 0.20 | 43.01 | 0.00 | 0.05 | 0.15 | 0.42 | 88.34 | 0.50 | 2.44 | 0.05 | 0.00 | 0.02 | 0.00 | 3.00 | 2.00 | 0.00 | 2.00 |
| 47 | 6.95 | 37.54 | 0.42 | 0.00 | 0.40 | 43.37 | 0.08 | 0.07 | 0.24 | 0.35 | 89.44 | 0.49 | 2.43 | 0.06 | 0.00 | 0.03 | 0.00 | 3.01 | 1.99 | 0.01 | 1.99 |
| 48 | 6.78 | 38.47 | 0.22 | 0.03 | 0.00 | 43.55 | 0.17 | 0.00 | 0.17 | 0.54 | 89.91 | 0.48 | 2.49 | 0.03 | 0.00 | 0.00 | 0.00 | 2.99 | 1.99 | 0.01 | 2.01 |
| 49 | 5.30 | 39.13 | 0.22 | 0.00 | 0.25 | 43.29 | 0.35 | 0.02 | 0.13 | 0.21 | 88.96 | 0.38 | 2.55 | 0.03 | 0.00 | 0.02 | 0.00 | 2.98 | 2.00 | 0.03 | 2.02 |
| 50 | 4.54 | 39.85 | 0.20 | 0.00 | 0.24 | 42.98 | 0.00 | 0.07 | 0.12 | 0.28 | 88.26 | 0.32 | 2.63 | 0.03 | 0.00 | 0.02 | 0.00 | 3.00 | 2.01 | 0.00 | 2.01 |
| K | - | 39.50 | - | - | - | 37.18 | - | - | - | - | 76.68 | - | 3.00 | - | - | - | - | 3.00 | 2.00 | - | 2.00 |
| x | y | z | Ueq (Å2) | |
|---|---|---|---|---|
| As | 0.31553(3) | 0 | 0.37387(7) | 0.0080(1) |
| (Zn,Co)1 | 0 | 0 | 0 | 0.0106(2) |
| (Zn,Co)2 | 0 | 0.38524(3) | 0 | 0.0104(2) |
| O1 | 0.1490(2) | 0 | 0.3751(5) | 0.0124(7) |
| O2 | 0.4051(3) | 0 | 0.7254(5) | 0.0119(7) |
| O3 | 0.34275(18) | 0.10662(13) | 0.2115(4) | 0.0121(5) |
| O4 | 0.09853(18) | 0.11482(13) | 0.8081(4) | 0.0144(5) |
| O5 | 0.39981(19) | 0.22666(15) | 0.7149(4) | 0.0186(5) |
| As–O1 | 1.710(3) | O3–As–O2(x2) | 110.08(7) | |
| As–O2 | 1.685(2) | O3i–As–O3(x2) | 116.33(12) | |
| As–O3(x2) | 1.6843(18) | O3–As–O1(x2) | 106.73(7) | |
| <As–O> | 1.691(2) | O2–As–O1 | 106.31(12) | |
| (Zn,Co)1–O1(x2) | 2.023(2) | O1– (Zn,Co)1–O1ii | 180.0 | |
| (Zn,Co)1–O4ii(x4) | 2.1718(18) | O1– (Zn,Co)1–O4ii(x4) | 87.46(7) | |
| <(Zn,Co)1–O> | 2.122(2) | O1– (Zn,Co)1–O4iv(x4) | 92.54(7) | |
| O4ii– (Zn,Co)1–O4iv(x2) | 180.00(11) | |||
| O4ii– (Zn,Co)1–O4v(x2) | 89.62(10) | |||
| O4iv– (Zn,Co)1–O4v(x2) | 90.38(10) | |||
| (Zn,Co)2–O2vi(x2) | 2.0883(17) | O2vi– (Zn,Co)2–O2ix | 84.97(10) | |
| (Zn,Co)2–O5vii(x2) | 2.1027(19) | O2vi– (Zn,Co)2–O5vii (x2) | 178.02(7) | |
| (Zn,Co)2–O3viii(x2) | 2.1171(18) | O2ix– (Zn,Co)2–O5vii (x2) | 93.10(8) | |
| <(Zn,Co)2–O> | 2.1027(18) | O5vii– (Zn,Co)2–O5vi | 88.84(11) | |
| O2vi– (Zn,Co)2–O3viii (x2) | 88.37(9) | |||
| O2ix– (Zn,Co)2–O3viii (x2) | 87.27(9) | |||
| O5vii– (Zn,Co)2–O3viii (x2) | 91.95(7) | |||
| O5vi– (Zn,Co)2–O3viii (x2) | 92.27(7) | |||
| O3viii– (Zn,Co)2–O3x | 174.08(10) | |||
| D—H···A | D—H | H···A | D···A | D—H···A |
|---|---|---|---|---|
| O4—H41···O5 i | 0.96 | 2.56 | 3.109 (3) | 116.1 |
| O4—H41···O5 ii | 0.96 | 2.38 | 2.899 (3) | 113.7 |
| O4—H43···O1 | 0.96 | 1.84 | 2.730 (3) | 153.4 |
| O5—H53···O4 iii | 0.96 | 2.01 | 2.899 (3) | 152.3 |
| O5—H54···O3 | 0.96 | 1.90 | 2.819 (3) | 159.4 |
| Member of the Solid Solution | Köttigite mol% | M1-O | M2-O | As-O | O-As-O |
|---|---|---|---|---|---|
| Erythrite [4] | 4 | 2.122(1) | 2.088(1) | 1.710(1) | 109.36(3) |
| Co-köttigite | 64 | 2.122(2) | 2.103(2) | 1.691(2) | 109.41(8) |
| Köttigite [5] | 81 | 2.115(5) | 2.100(5) | 1.682(5) | 109.4(2) |
| ER2 | Erythrite [5] | Hof | Na31 | Na27 | ER1 | Na29 | Köttigite [6] | |
|---|---|---|---|---|---|---|---|---|
| a, Å | 10.2591(6) | 10.2480 | 10.2657(9) | 10.2657(1) | 10.2669(2) | 10.2458(1) | 10.2567(1) | 10.241(3) |
| b, Å | 13.4091(6) | 13.4249 | 13.4355(8) | 13.4264(2) | 13.4240(2) | 13.4196(1) | 13.4247(2) | 13.405(3) |
| c, Å | 4.7635(6) | 4.7559 | 4.7640(6) | 4.7616(1) | 4.7626(1) | 4.7580(9) | 4.7575(1) | 4.757(2) |
| βo | 105.077(9) | 105.1116 | 105.126(2) | 105.1379(3) | 105.1372(4) | 105.162(2) | 105.1658(4) | 105.21(2) |
| V, Å3 | 632.739(7) | 631.680 | 634.305(7) | 633.519 | 633.627(3) | 631.439(2) | 632.265(2) | 630.168 |
| Co/(Co + Zn) | 1.00 | 0.96 | - | 0.71 | - | 0.60 | 0.35 | 0.15 |
| Co (apfu) | 2.72 (n = 28) | 2.78 | Co >> Zn | 2.04 (n = 75) | Co>Zn | 1.69 (n = 117) | 1.00 (n = 52) | 0.42 |
| Zn (apfu] | 0.01 (n = 28) | 0.11 | 0.83 (n = 75) | 1.11 (n = 117) | 1.87 (n = 52) | 2.44 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciesielczuk, J.; Dulski, M.; Janeczek, J.; Krzykawski, T.; Kusz, J.; Szełęg, E. Crystal Chemistry of an Erythrite-Köttigite Solid Solution (Co3–xZnx) (AsO4)2·8H2O. Minerals 2020, 10, 548. https://doi.org/10.3390/min10060548
Ciesielczuk J, Dulski M, Janeczek J, Krzykawski T, Kusz J, Szełęg E. Crystal Chemistry of an Erythrite-Köttigite Solid Solution (Co3–xZnx) (AsO4)2·8H2O. Minerals. 2020; 10(6):548. https://doi.org/10.3390/min10060548
Chicago/Turabian StyleCiesielczuk, Justyna, Mateusz Dulski, Janusz Janeczek, Tomasz Krzykawski, Joachim Kusz, and Eligiusz Szełęg. 2020. "Crystal Chemistry of an Erythrite-Köttigite Solid Solution (Co3–xZnx) (AsO4)2·8H2O" Minerals 10, no. 6: 548. https://doi.org/10.3390/min10060548